Novel ferulic acid esterase and applications thereof
A ferulic acid esterase, a new type of technology, applied in the field of genetic engineering, can solve problems such as defects in enzymatic properties and restrictions on the wide application of ferulic acid esterase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Establishment of metagenomic library and acquisition of positive clones, gene cloning and expression
[0040] 1. Extraction of total DNA
[0041] Weigh 6g sample, add 13.5ml DNA extraction buffer (0.1M Tris, 0.1M EDTA-Na, 0.1M Na 3 PO 4 , 1.5M NaCl, 1% CTAB, pH 8.0), shake vigorously for 3-5min, add 200μL lysozyme (100mg / ml), invert repeatedly 5-6 times, 37℃ water bath for 30min, add 1.5ml 20% SDS, 65℃ Water bath for 1h (during this period, turn it upside down several times every 15min), centrifuge at 8000r / min for 5min, take the supernatant, extract twice with an equal volume of chloroform, centrifuge at 16000r / min for 10min, take the supernatant, add 0.6 times the volume of isopropyl Alcohol, place at room temperature for 2h, centrifuge at 20000r / min for 20min, discard the supernatant, add 5mL of pre-cooled 70% ethanol to the pellet, centrifuge at 20000r / min for 10min, collect the DNA precipitate, air-dry, and dissolve with an appropriate amount of TE buff...
Embodiment 2
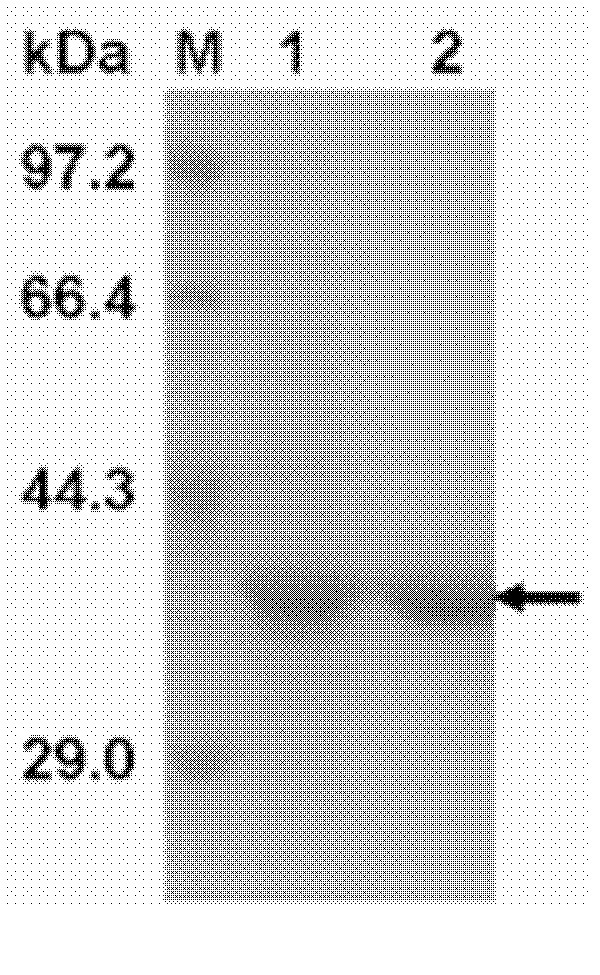
[0062] Embodiment 2 recombinant ferulic acid esterase Est27 enzyme activity assay
[0063] 1. Determination of enzyme activity
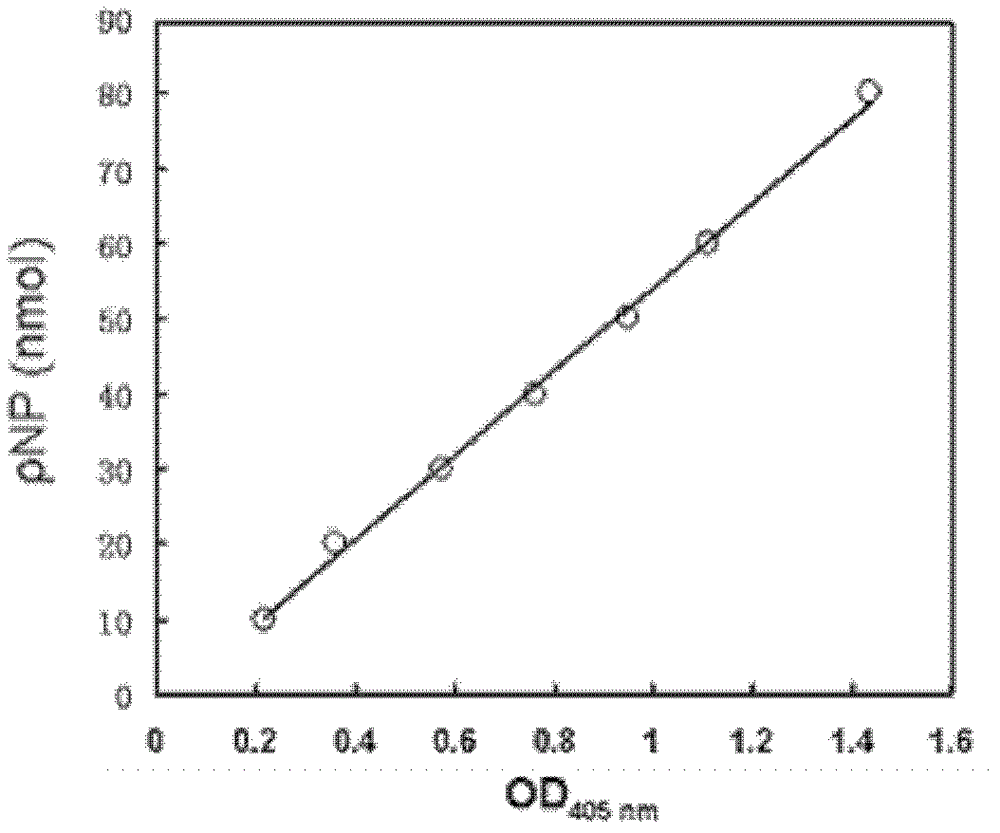
[0064] The invention uses p-nitrophenol ferulate as a substrate to detect the enzyme activity of ferulic acid esterase. The reaction system was 500 μl, which consisted of 50 μl of 10 mM p-nitrophenol ferulate stock solution (dissolved in DMSO), 440 μl of 0.1 mM potassium phosphate buffer containing 2.5% (v / v) Triton X-100 ( pH 6.8) and 10 μl enzyme solution. The reaction system was reacted at 40° C. for 5 minutes, and then the absorbance of p-nitrophenol released during the process was measured at a wavelength of 405 nm, and a blank control without adding enzyme solution was made. Determine its concentration according to the standard curve of p-nitrophenol. The enzyme activity unit is defined as: under the reaction conditions, the amount of enzyme required to catalyze 1 μmol of p-nitrophenol ferulate per minute is defined as 1 enzyme activity unit...
Embodiment 3
[0072] Example 3 Research on the enzymatic properties of recombinant ferulic acid esterase Est27
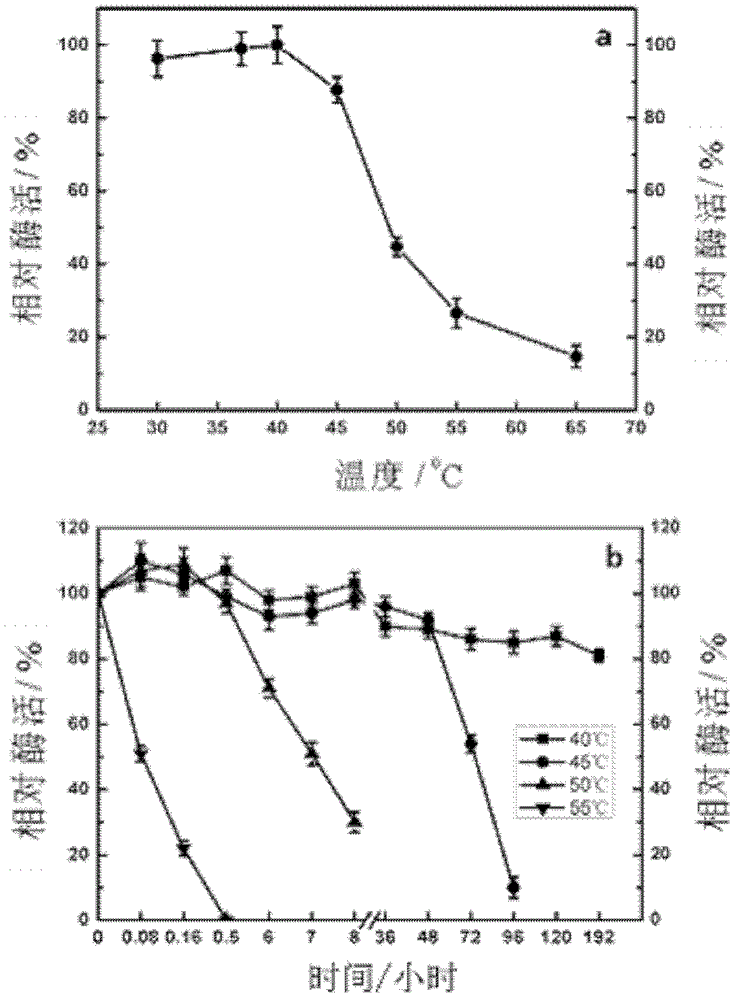
[0073] 1. Optimum reaction temperature and thermal stability of recombinant ferulic acid esterase Est27
[0074] After carrying out the enzymatic reaction of the crude enzyme liquid of recombinant ferulic acid esterase Est27 at 30-65° C., its enzyme activity was measured according to the above-mentioned method to obtain its optimum reaction temperature (recorded as 100% when the enzyme activity is the highest). In 100mM phosphate buffer (pH6.8), incubate the ferulic acid esterase Est27 enzyme solution at different temperatures (40-60°C), take out the enzyme solution at regular intervals and measure the residual enzyme activity to treat for 0min The enzyme activity of the enzyme solution is 100%. The test results are attached image 3As shown, the optimal reaction temperature of the recombinant protein Est27 is 40°C. The enzyme is stable at 40°C and has a half-life of 72h at 45...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com