Vulcanized aspartic acid modified melatonin derivative and application thereof
A compound, halogen technology, applied in the field of tumor drugs, can solve the problems of inconvenient administration, no curative effect, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: In this embodiment, compounds represented by formulas 2-5 and metal salts thereof were synthesized respectively.
[0059] a. the synthesis of the melatonin magnesium salt / zinc salt modified by aspartic acid sulfide of formula 2
[0060] In this example, the compound of formula 2 was synthesized according to the scheme shown below:
[0061]
[0062]
[0063] Step 1. Synthesis of sulfurized aspartic acid: (1) 60% NaH (1.8mmol) is suspended in THF (5ml) at 0-4°C, and 1 equivalent of methylphosphorous acid (CH 3 -SO 2 -H 2 PO 3 ), 1 equivalent of aspartaldehyde (commercial product, purchased from Sigma- Eldrich Company, compound 1a) was added therein, stirred at room temperature for 30 minutes, then added 1 equivalent of acetaldehyde (aldehyde), and continued to stir at room temperature for 120 minutes to generate aspartic acid sulfide (compound 1b) with a protective agent; (2) add 1 equivalent of trifluoroacetic acid (TAF) to react at 0-4°C for 60 ...
Embodiment 2
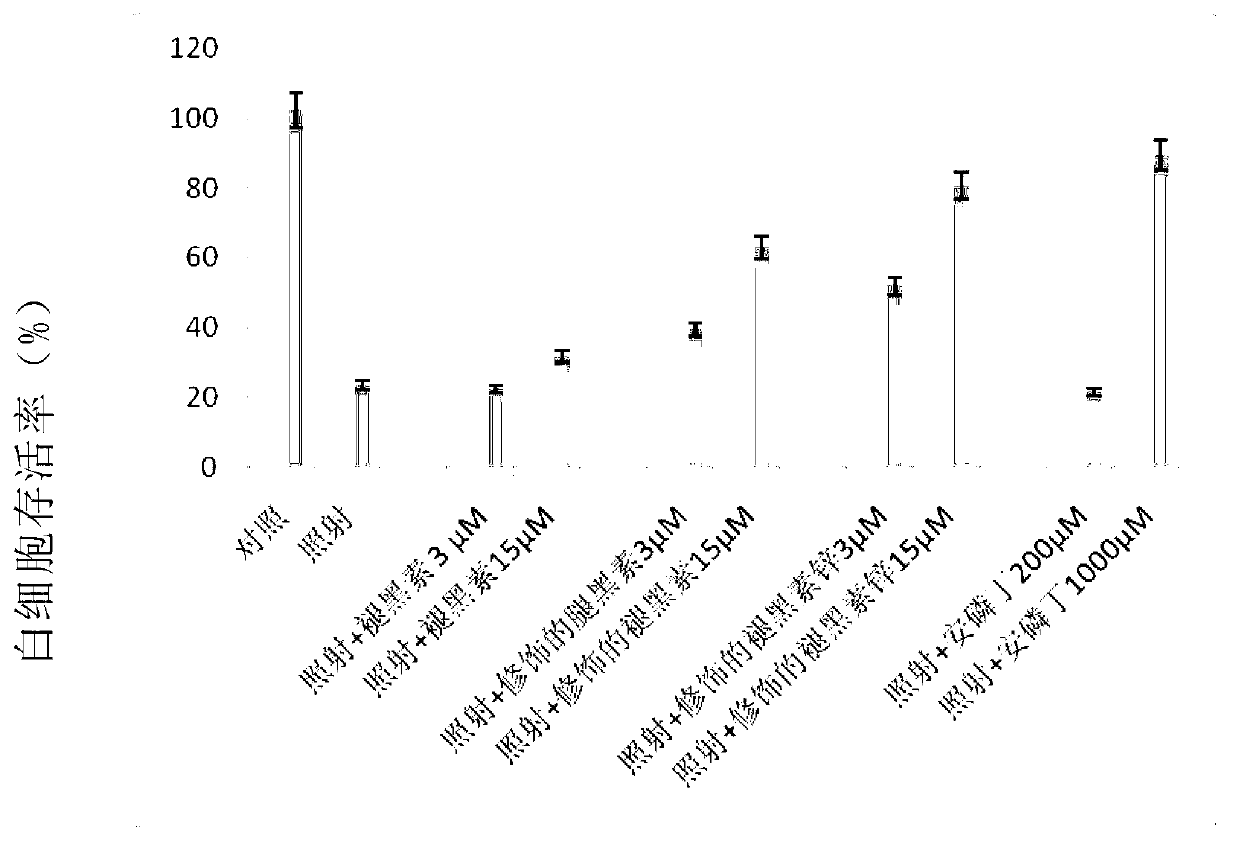
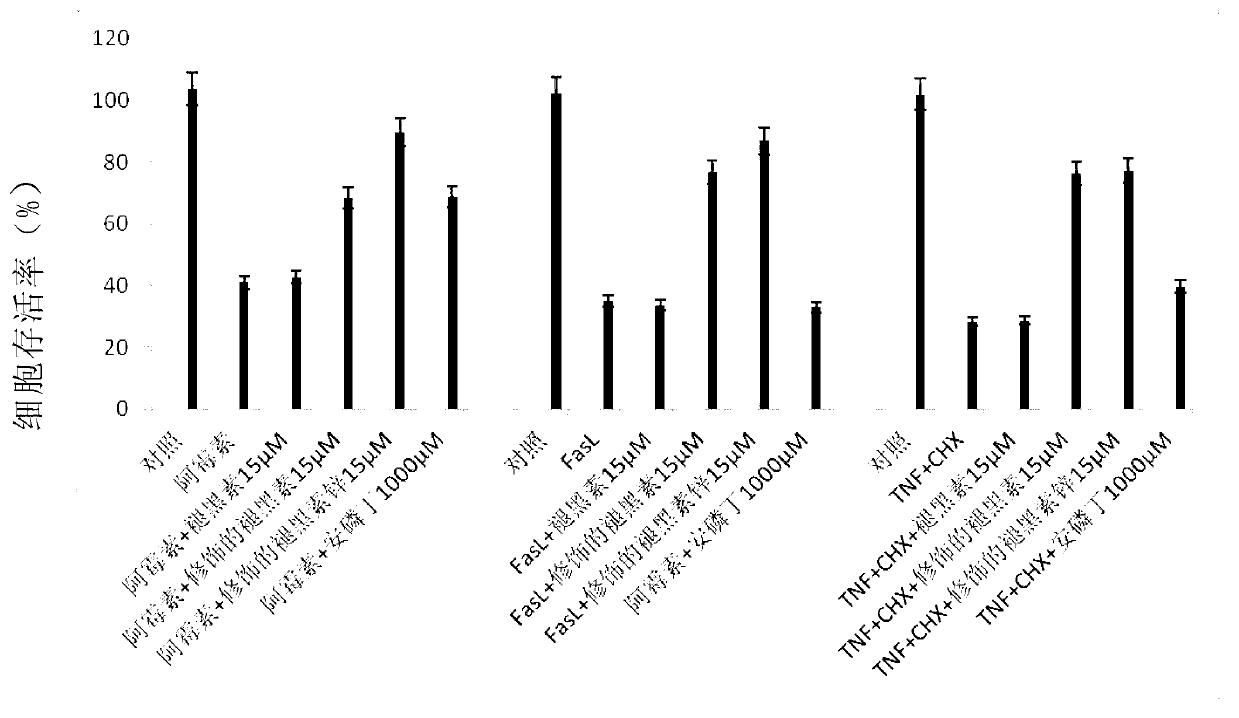
[0075] Example 2: Experiment of the protective effect of the compound synthesized in Example 1a and its zinc salt as a protective agent against human peripheral leukocyte damage caused by cobalt-60γ-rays.
[0076] In this example, isolated healthy human peripheral leukocytes (lymphocytes) were cultured in 96-well cell culture plates in RPMI1640 medium (containing 10% newborn calf serum, 1000 IU / ml recombinant human interleukin-2, 1% penicillin , 1% streptomycin) in conventional culture. Melatonin (raw material 2a used to prepare the compound of formula 2 in Example 1a), and aspartic acid melatonin sulfide (compound 2b) and aspartic acid melatonin zinc sulfide prepared in Example 1a (compound 3b), amifostine (a protective agent that has been used clinically as a positive control) was added as a protective agent to human peripheral leukocytes cultured in vitro, and the final concentrations were: melatonin (3μM and 15μM) and asparagus sulfide melatonin (3 μM and 15 μM), zinc asp...
Embodiment 12
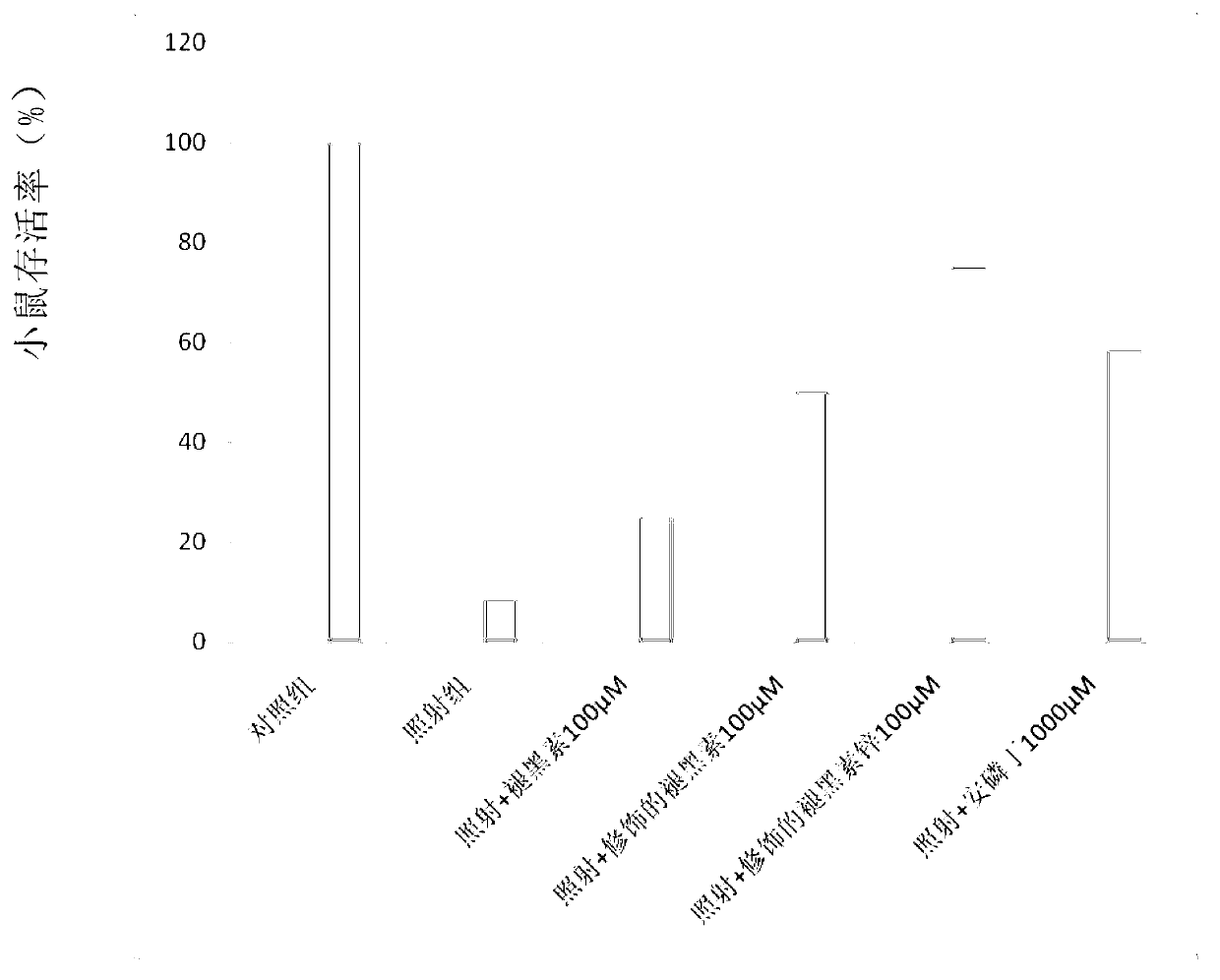
[0098] Example 12 Indomethacin combined with the protective agent of the present invention has the tumor inhibitory effect and normal tissue selective effect on γ-ray-induced transplanted tumors in nude mice (in vivo experiment)
[0099] In this example, the product synthesized in Example 1a (ie, the zinc salt of the compound of formula 2) was used for experiments. Under sterile conditions, the breast cancer cell (MCF-7) suspension in the logarithmic growth phase (adjust the cell concentration to 1×10 with normal saline) 7 w / mL) was inoculated subcutaneously on the right axillary back of experimental female nude mice. 6 / only. After 10 days, a 4-6 mm subcutaneous transplanted tumor appeared. The tumor-bearing nude mice were randomly divided into groups with 3 mice in each group. (1) Control group, without any treatment; (2) Irradiation group, without any protective agent; (3) Radiation therapy + melatonin zinc aspartate sulfide; (4) Radiation therapy + indomethacin; (5) Ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Survival rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com