Preparation method of WO3/ZrO2 solid super acidic catalyst
A solid super acid and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., to achieve enhanced catalytic activity, low reaction temperature, and small specific surface area. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
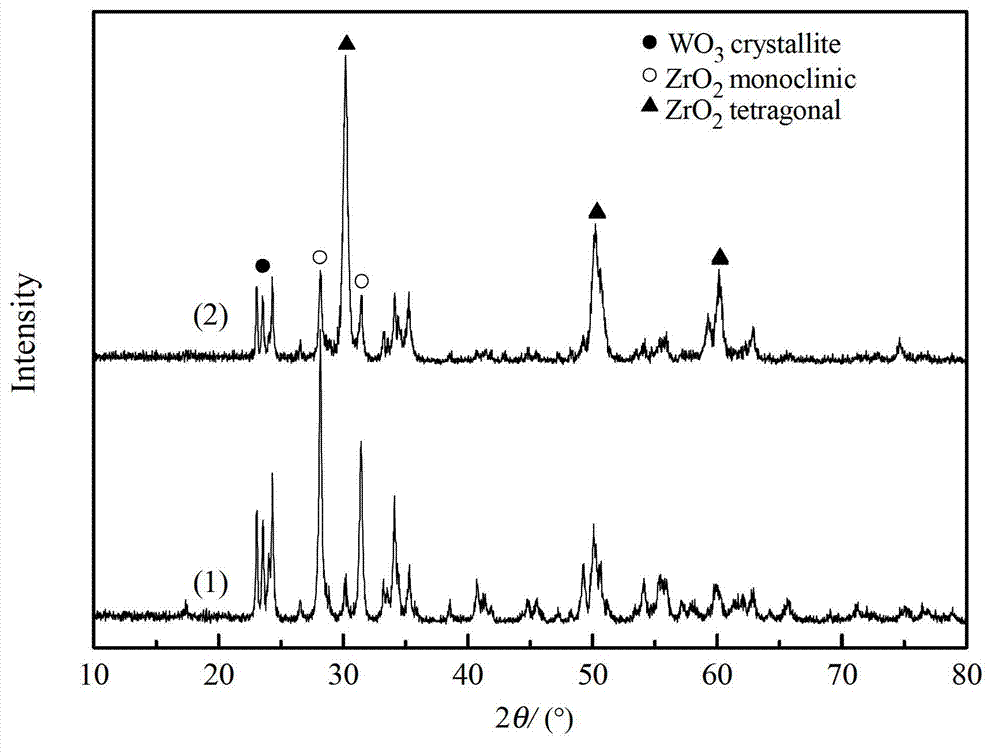
Image
Examples
Embodiment 1
[0018] Example 1. Under the condition of stirring, add 0.6 m 3 min -1 25% volume fraction of concentrated ammonia water was added dropwise at a high speed to form a hydrogel, and the pH value was adjusted to 9~10. Stirring was continued for 1-2 hours, and after aging at room temperature for 1-12 hours, the prepared hydrogel was transferred to a round-bottomed flask connected with a condenser tube, and heated at 90-110°C for 1-12 hours. Suction filter and wash the precipitate with deionized water until there is no chloride ion. Then filter the cake in a constant temperature drying oven with a concentration of 99% C 2 h 5 Soak in OH for 1~12 hours. Take out after cooling to room temperature, filter to remove residual C 2 h 5 OH, dry at 90~120°C for 1~12 hours, grind it finely to get white powder zirconium hydroxide carrier. Then impregnate Zr(OH) with ammonium metatungstate solution (W mass fraction is 15%) 4 , calcined at 800°C for 2 to 5 hours under a static air atmos...
Embodiment 2
[0024] The catalyst was prepared according to the method of Example 1, except that the mass fraction of W in the mixture was 10%. The conversion rate of n-heptane catalyzed by the obtained catalyst and the selectivity of isoheptane were shown in Table 3.
[0025] n-heptane conversion Isoheptane selectivity Product yield 38.56 95.83% 36.95%
[0026] table 3
[0027] Note: In the evaluation of reaction performance, samples were taken for analysis after 20 minutes of stable reaction, and the test time was all over 48 hours.
Embodiment 3
[0029] The catalyst was prepared according to the method of Example 1, except that the mass fraction of W in the mixture was 18%. The conversion rate of n-heptane catalyzed by the obtained catalyst and the selectivity of isoheptane were shown in Table 4.
[0030] n-heptane conversion Isoheptane selectivity Product yield 32.81% 90.68% 29.75%
[0031] Table 4
[0032] Note: In the evaluation of reaction performance, samples were taken for analysis after 20 minutes of stable reaction, and the test time was all over 48 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com