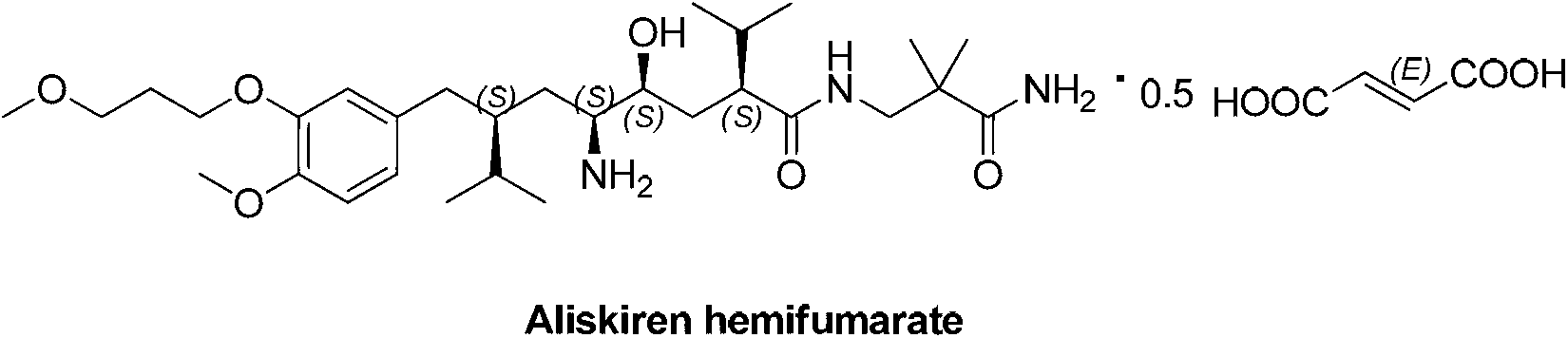
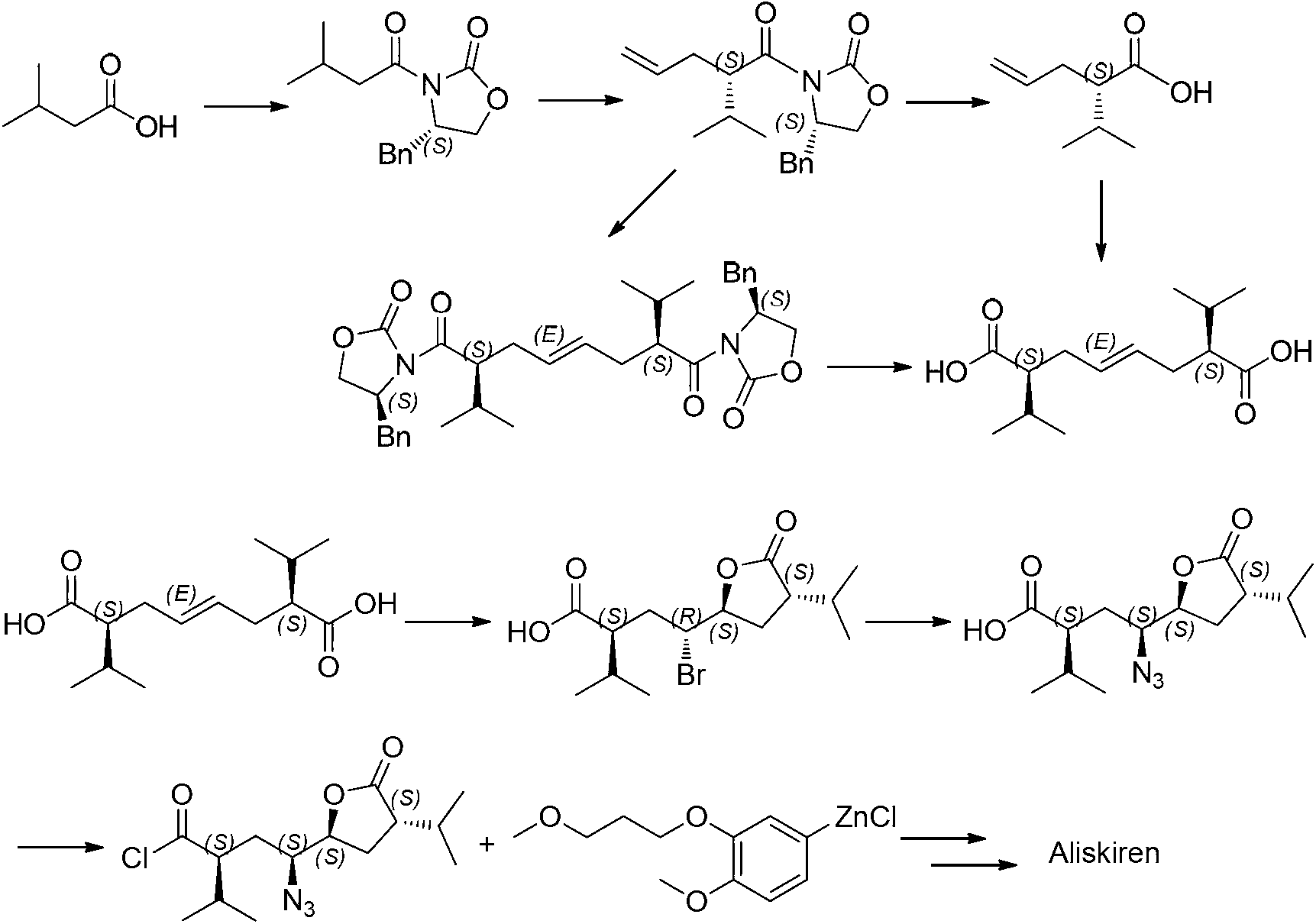
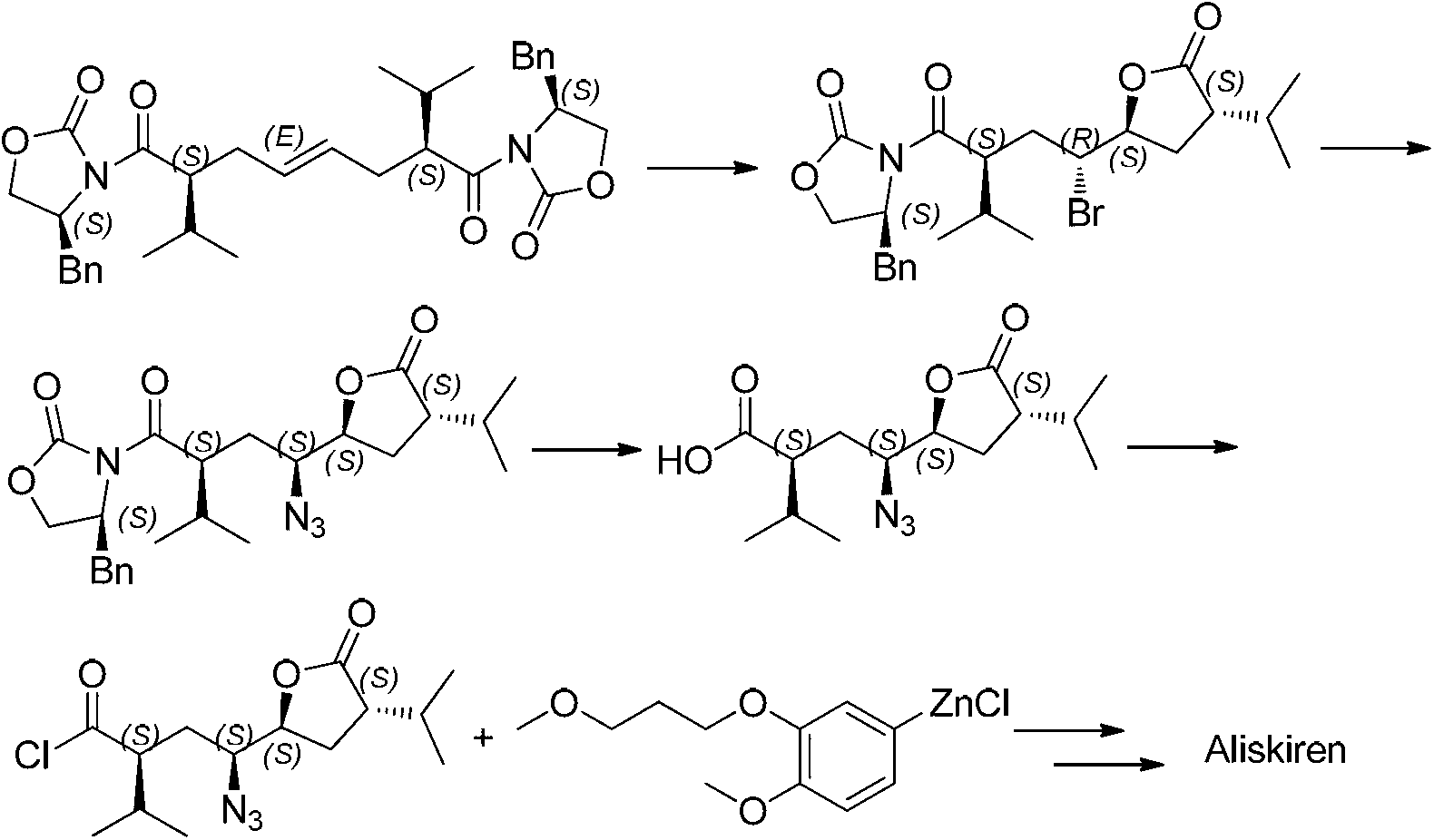
1,8-dicarbonyl-4,5-epoxy compound and preparation method thereof
An epoxy compound and dicarbonyl technology, applied in the field of aliskiren intermediate 1,8-dicarbonyl-4,5-epoxy compound and its preparation, can solve the problem of poor selectivity, poor chiral selectivity and poor yield. higher question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
[0074] N 2 Under protection, in a 500mL three-necked flask, dissolve compound 4 (26.83g, 102.7mmol) in 80mL of anhydrous THF, and add 1.0M LiHMDS (110mL, 110mmol) dropwise at low temperature (-50°C). After the addition is complete, - Stir at 50°C for 1 hour, then add 1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone (DMPU) (28.95g, 225.8mmol) dropwise. Stir at 50°C for 0.5h, then add cis-1,4-dibromo-2-butene 5 (8.78g, 41.0mmol) in 18mL of anhydrous THF solution, stir at -50°C for 1h, then naturally warm to 0°C, Keep it at 0°C for 1h, then raise it to 5-10°C for 1.5h, and track it with TLC until the end of the reaction. Saturated with 50 mL of NH 4 Quench the reaction with Cl solution, remove THF by rotary evaporation, extract the aqueous phase with (30mL×3) ethyl acetate, combine the EA phases, wash the EA phase with 1M (50mL×4) dilute HCl for about 3 times, wash the EA phase with anhydrous MgSO 4 Dry, spin dry, drain the solvent, and recrystallize the obtained cru...
Embodiment 2
[0077]
[0078] Add compound 2.3 (287.15g, 0.5mol), tetrahydrofuran (300mL), water (100mL), lithium hydroxide monohydrate (33.56g, 0.8mol) in sequence in a 1L three-necked flask, and add 30% hydrogen peroxide dropwise at 0-5°C (247.2mL, 2.4mol), after reacting at room temperature for 3 hours, at 0°C, dropwise add a saturated aqueous solution of sodium bisulfite (250.00g, 2.4mol) to quench the reaction, adjust the pH of the system to 10 with 6M aqueous sodium hydroxide solution, and spin The tetrahydrofuran was evaporated, the aqueous phase was washed with ethyl acetate (3×100 mL), the pH of the system was adjusted to 2 with 6M hydrochloric acid at 0° C., the aqueous phase was extracted with ethyl acetate (3×100 mL), the organic phases were combined, and dried over anhydrous magnesium sulfate. After filtration, the organic phase was removed by rotary evaporation to obtain 117.76 g of light yellow viscous liquid, which was the target compound 6, with a yield of 92%.
[0079] ...
Embodiment 3
[0081]
[0082] In a 250mL one-mouth bottle, add 60mL of methanol and compound 6 (11.70g, 45.66mmol) in sequence, add thionyl chloride (16.31g, 136.98mmol) dropwise at 0°C, and reflux for 4 hours after dropping, and cool to room temperature. Remove the organic phase by rotary evaporation, add ethyl acetate (100 mL), wash the organic phase with saturated aqueous sodium bicarbonate (20 mL), saturated brine (20 mL), and water (20 mL) successively, dry the organic phase with anhydrous magnesium sulfate, filter, Rotary evaporation gave 11.52 grams of light brown liquid, which was the target compound 2.1,
[0083] Yield 89%.
[0084] 1 H NMR (500MHz, CDCl 3 )δ5.42(d,J=3.7Hz,2H),3.71(s,6H),2.23(m,4H),1.88(dd,J=13.6,6.8Hz,2H),0.96(dd,J=18.4 ,6.7Hz,12H). MS-ESI: m / z(%)=285.2(100)[M+H] + . [α]D=-9.1°(c=1.0,CH 2 Cl 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com