High-dose bacillus licheniformis viable bacterium composition as well as preparation method and application thereof
The technology of Bacillus licheniformis and the composition is applied in the field of microorganisms, which can solve the problems of reducing the fluidity of preparation products, difficult to achieve industrialized production, and low viable bacteria content, and achieves recovery of intestinal colonization resistance, fast onset, and viable bacteria content. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Materials and methods
[0019] 1.1 Materials
[0020] 1.1.1 Experimental animals
[0021] SPF-grade Kunming mice, age 6-8 weeks, weight 20±2g, male and female, half male and half male, provided by the animal room of Shenyang No. .
[0022] 1.1.2 Drugs
[0023] Ampicillin sodium for injection (Zhongnuo Pharmaceutical (Shijiazhuang) Co., Ltd., 0.5g / bottle), Bacillus licheniformis BL63516 bacterial powder (Northeast Pharmaceutical Group Shenyang First Pharmaceutical Factory, batch number: YJ12018, 29.4 billion / g)
[0024] 1.1.3 Main reagents
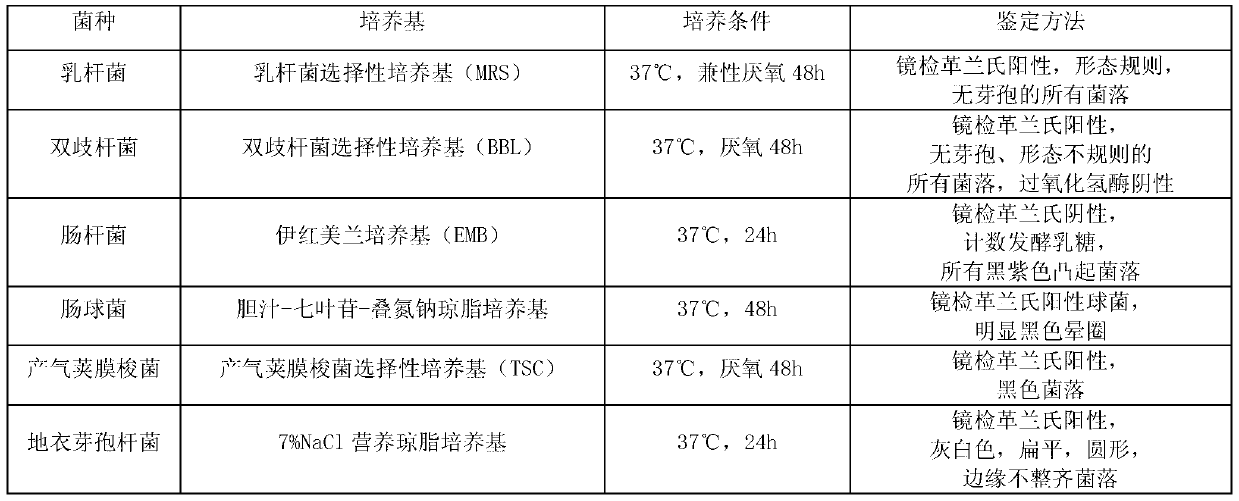
[0025]Bifidobacterium Selective Medium (BBL), Enterococcus Selective Medium (Bile-Escin-Sodium Azide Agar), Lactobacillus Selective Medium (MRS), Enterobacterial Medium (EMB), Gas Production Clostridium perfringens culture medium (TSC) was purchased from Beijing Land Bridge Technology Co., Ltd. Beef extract and peptone were purchased from Beijing Aoboxing Biotechnology Co., Ltd., and sodium chloride was purchased from Tianji...
Embodiment 2
[0068] 1 Materials and methods
[0069] 1.1 material is the same as embodiment one
[0070] 1.2 Method
[0071] 1.2.1 Bacillus licheniformis BL63516 bacterial suspension preparation
[0072] Dissolve 5g of bacterial powder in 600ml of normal saline to prepare a high-concentration bacterial solution of 8.33mg / ml. Take 500ml of high-concentration bacterial solution and add 250ml of normal saline to prepare a 5.56mg / ml bacterial solution. Carry out 2-fold serial dilution to 2.78mg / ml, 1.39mg / ml bacterial solution.
[0073] It needs to be prepared and fed before each gavage.
[0074] 1.2.2 Grouping of test animals
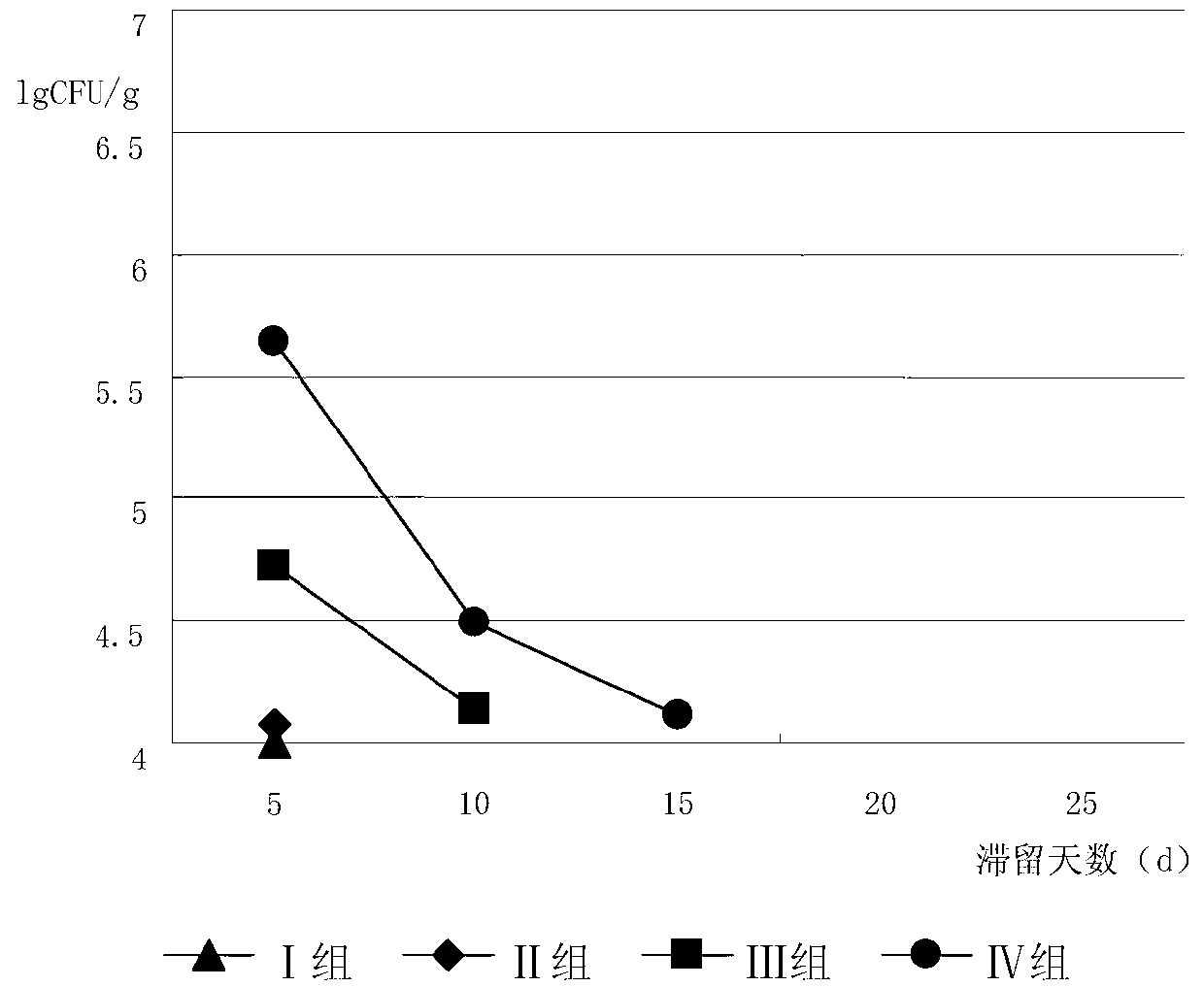
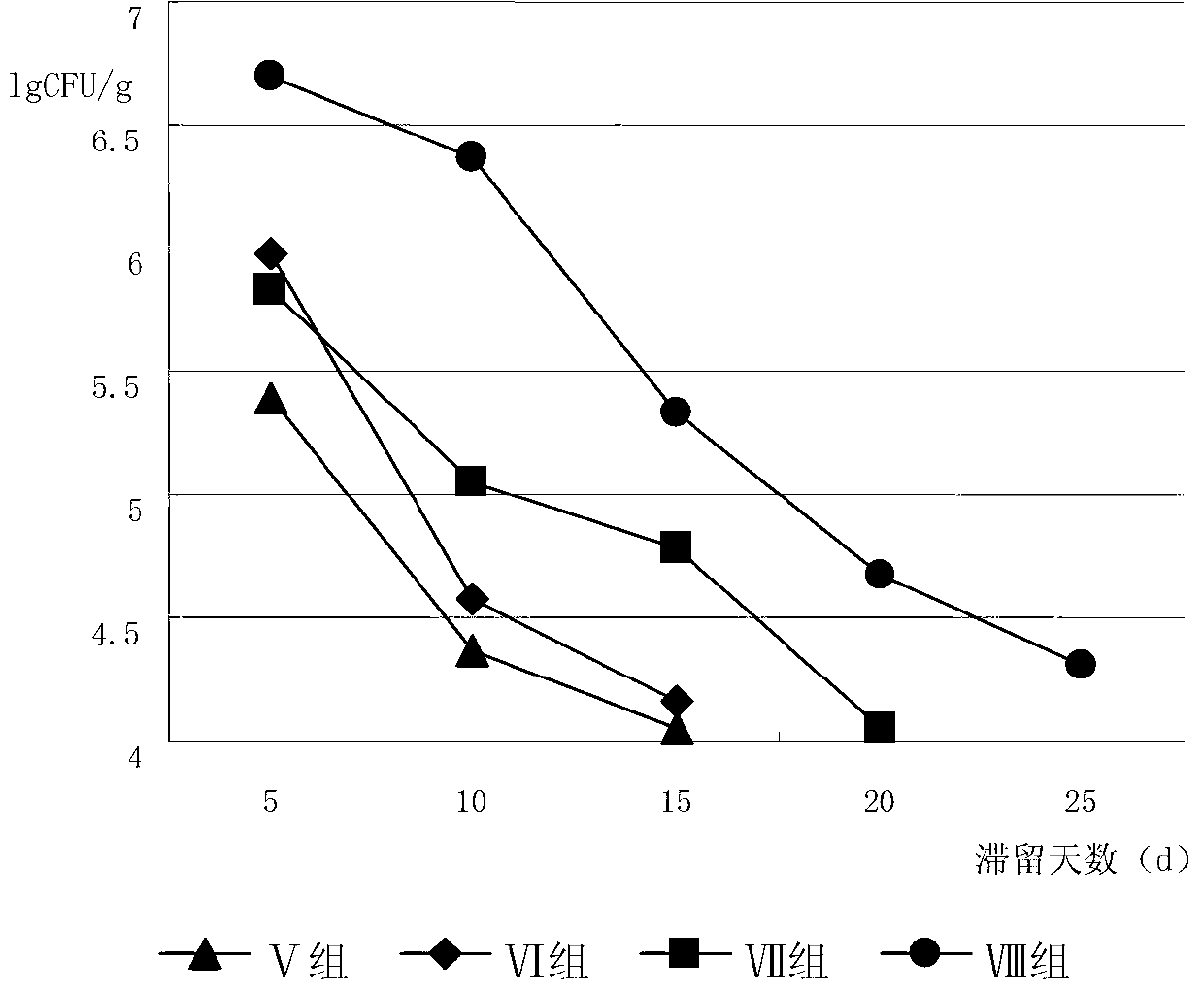
[0075] 72 healthy Balb / c mice were selected, weighing 20±2g. After feeding with mouse feed and clean water for 1 week, the mice were randomly divided into 6 groups, 12 in each group, half male and half male. Group 1 was the blank control group, and the other 5 model groups were made with ampicillin sodium to make the diarrhea model of intestinal flora imbalance, an...
Embodiment 3
[0099] MnSO 4 Concentrations were 0%, 0.005%, 0.01%, 0.02%, 0.03%, 0.04%, 0.05%, 0.06%, 0.07%.
[0100] Take a ring of Bacillus licheniformis BL63516 with a sterile hook, inoculate the nutrient broth medium, and cultivate it at 36-38°C for 9 hours to make a seed solution. Inoculate the seed liquid into the shake flask nutrient broth medium, the inoculum amount is 0.1%, the liquid volume is 200ml / 1000ml, cultivate at 37°C and 200r / min for 18-20h, then add different concentrations of manganese ions to the fermentation liquid, and cultivate to 22 -24h. The bacteria sludge was collected by centrifugation, and the vegetative body was killed by heating at 62.5°C for 15 minutes, and then 10-fold serial dilution was performed to measure the number of spores in the fermentation broth. The results are shown in Table 12.
[0101] Table 12 Manganese ion concentration test results (spore number unit: 10 7 CFU / mg)
[0102] Manganese ion concentration
[0103] The results showe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viable count | aaaaa | aaaaa |
| Viable count | aaaaa | aaaaa |
| Viable count | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com