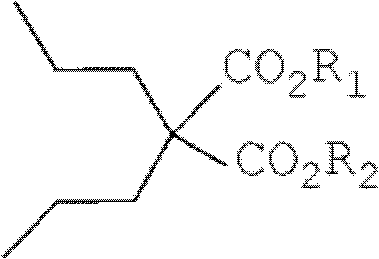
Dipropylmalonic acid diester preparation method
A technology of dipropylmalonate diester and malonate diester, which is applied in the field of chemical pharmacy, can solve problems such as troublesome post-processing, impact on product quality and yield, poor solubility, etc., and achieve simplified reaction and post-processing Effects of treatment steps, simplification of post-processing steps, and improvement of reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
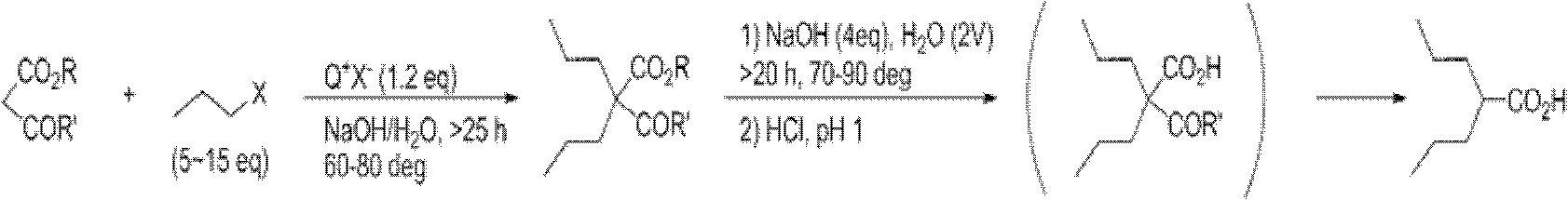
[0049] According to one embodiment of the present invention, a kind of preparation method of dipropylmalonate diester is provided, it comprises:
[0050] 1) in organic reaction solvent, take alkali as catalyzer, make diester of malonate and 1-halogenated n-propane react, thereby make dipropyl malonate diester, its reaction formula is as following reaction formula (I ) as shown:
[0051]
[0052] Reaction formula (I)
[0053] Among them, R 1 , R 2 each independently selected from straight-chain or branched-chain alkanes with 1-5 carbon atoms, preferably straight-chain or branched-chain alkanes with 1-3 carbon atoms, more preferably methyl or ethyl, and preferably, all R 1 , R 2 Same; X is a halogen, preferably chlorine, bromine, or iodine, more preferably bromine; the base is MO-R 3 , where R 3 It is a linear or branched chain alkane with 1-4 carbon atoms, and M is sodium or potassium.
[0054] Preferably, the reaction is carried out under heating to reflux.
[0055...
Embodiment 1
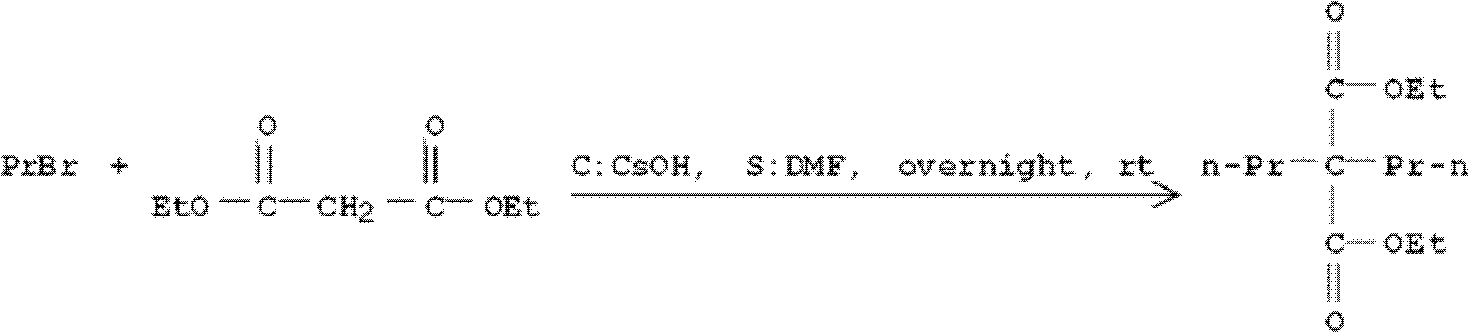
[0076] Potassium tert-butoxide is used as a base and tert-butanol is used as a reaction solvent to prepare diethyl 2,2-dipropylmalonate.
[0077]
[0078] Add 50mL of tert-butanol to a 500mL three-necked flask, add 10g (62.5mmol, 1.0eq) of diethyl malonate, 15.3g (125mmol, 2.0eq) of 1-bromopropane, and 14g (125mmol, 2.0eq) of potassium tert-butoxide under stirring. eq), be warming up to reflux, add 1-bromopropane 3.8g (31.8mmol, 0.5eq) and Potassium tert-butoxide 3.5g (31.8mmol, 0.5eq) after 5 hours, take a sample TLC detection reaction completely again after 3 hours, After cooling down to room temperature, filter with suction, wash the filter cake with 50 mL of ethyl acetate, combine the filtrate, wash the filtrate twice with tap water (50 mL×2), wash the filtrate twice with saturated sodium chloride aqueous solution (50 mL×2), and dry over anhydrous sodium sulfate , and the filtrate was spin-dried to obtain 14.06 g of crude product, the crude product yield was 92%, and it...
Embodiment 2
[0080] Sodium ethoxide is used as a base, and ethanol is used as a reaction solvent to prepare diethyl 2,2-dipropylmalonate.
[0081]
[0082] Add 60 mL of ethanol to a 500 mL three-necked flask, add 10 g (62.5 mmol, 1.0 eq) of diethyl malonate, 15.3 g (125 mmol, 2.0 eq) of 1-bromopropane, and 8.5 g (125 mmol, 2.0 eq) of sodium ethylate while stirring, Raise the temperature to reflux, add 3.8g (31.8mmol, 0.5eq) of 1-bromopropane and 2.1g (31.8mmol, 0.5eq) of sodium ethoxide after 5 hours, monitor the reaction by TLC for another 4 hours, and filter it after cooling down to room temperature , the filter cake was washed with 50 mL of ethyl acetate, the combined filtrates were washed with tap water twice (50 mL × 2), the filtrate was washed twice with saturated aqueous sodium chloride solution (50 mL × 2), dried over anhydrous sodium sulfate, and the filtrate was spin-dried to obtain The crude product was 12.77g, the crude product yield was 83.6%, and it could be directly used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com