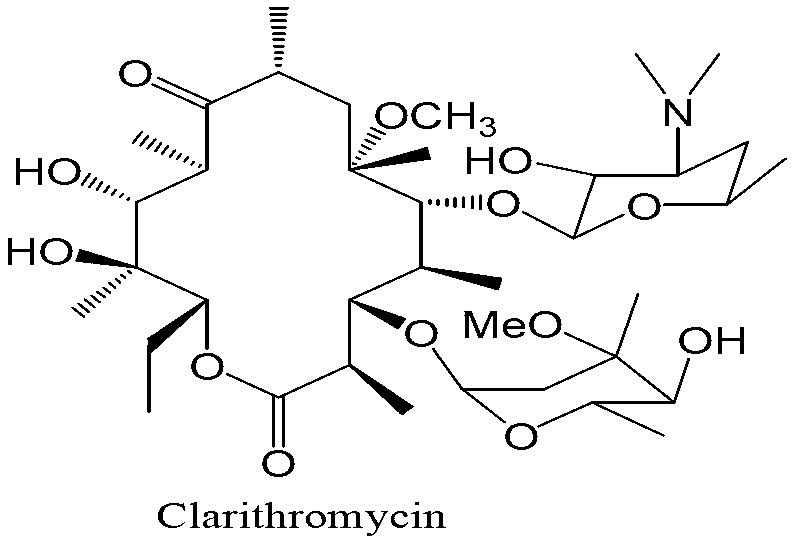
A preparation process of a clarithromycin impurity O or similar compounds
A technology of clarithromycin and preparation process, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of less reports on the preparation process of impurities, and achieves high product yield, good purity, and easy production. The effect of crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
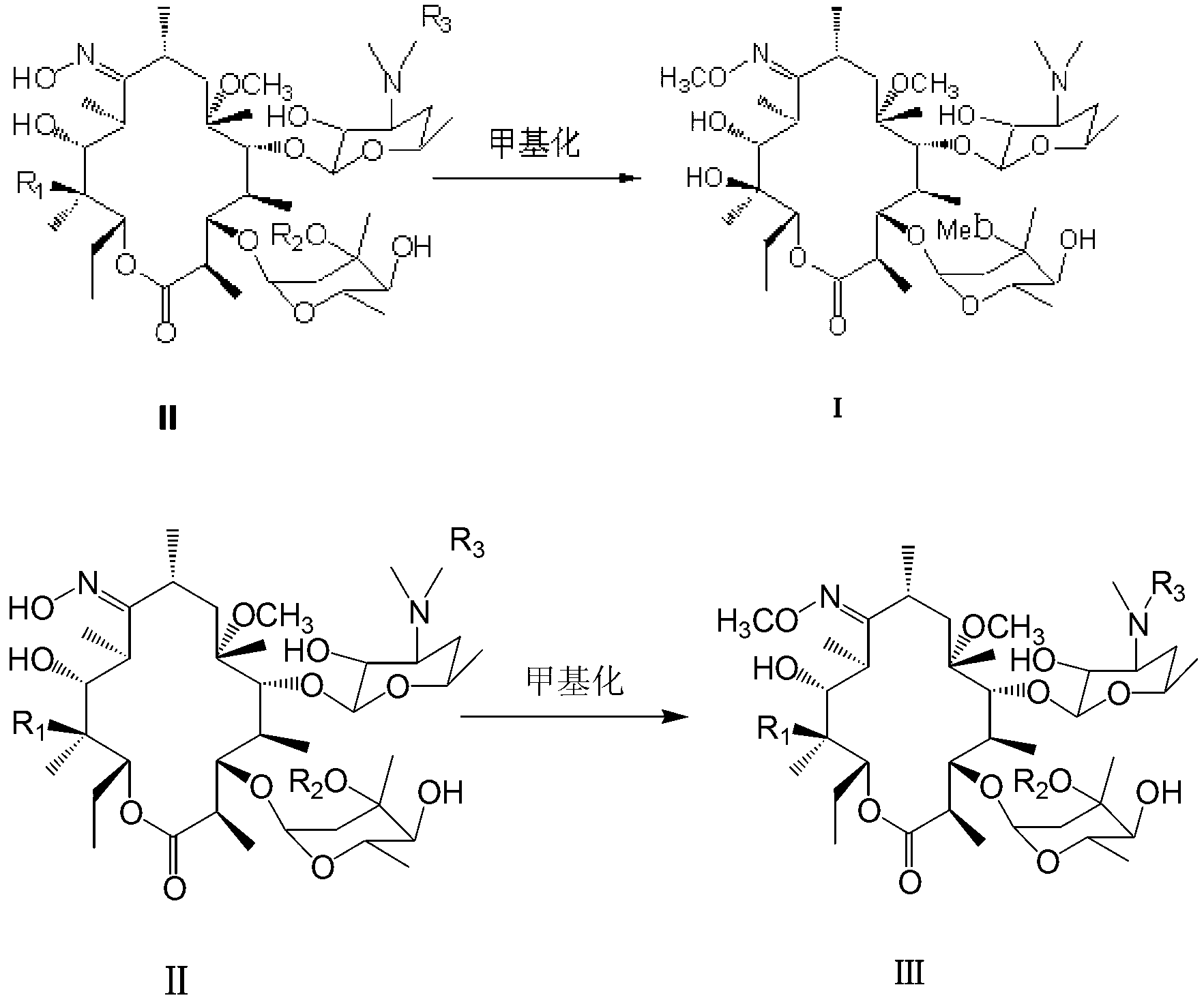
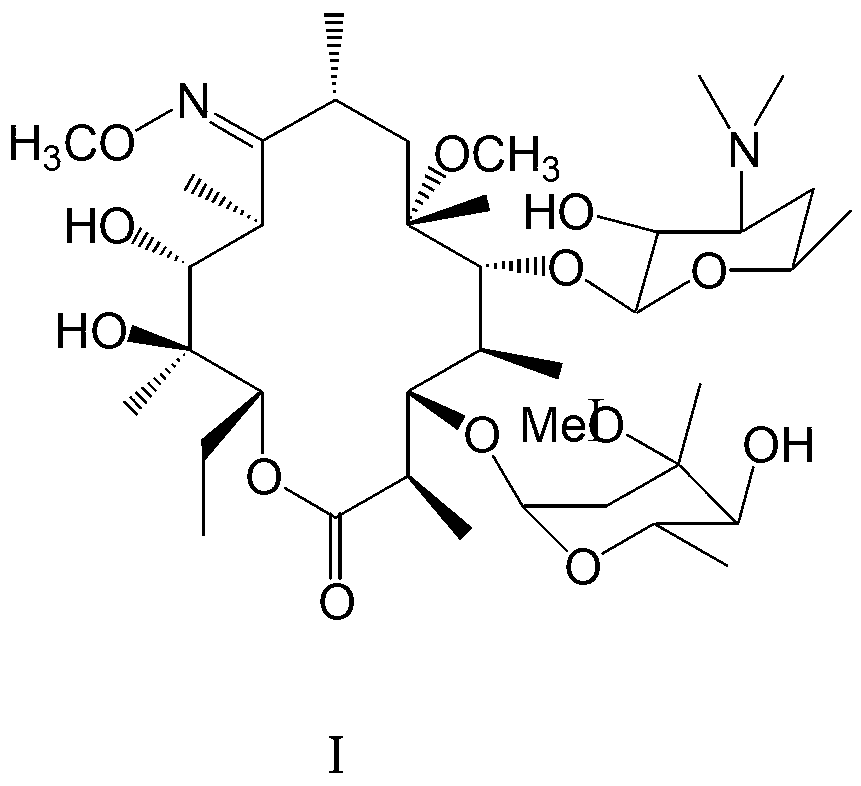
[0021] In a 250ml reaction flask, add clarithromycin oximide 10g (13.4mmol), methanol 150ml., stir to dissolve, add 3.82g (40mmol) methyl bromide, sodium hydroxide 1.6g (40mmol), react at 80°C for 1 hour, and react Finished, filter with suction, concentrate the filtrate under reduced pressure at 40°C, remove methanol, dissolve the residue with 20ml of acetone, control the temperature at 35°C, slowly add 30ml of water dropwise, until the solid precipitates, keep warm for 1 hour, drop to 8°C, and filter with suction , and the filter cake was vacuum-dried at 45°C to obtain 3.8 g of white solid I. Yield 38%, content 98.6%.
Embodiment 2
[0023] In a 250ml reaction flask, add 10g (13.4mmol) of clarithromycin oxime, 100ml of methylene chloride, stir to dissolve, add 3.32g (20mmol) of potassium iodide, 1.68g (20mmol) of sodium bicarbonate, and react at 0°C for 24 hours , the reaction was completed, suction filtered, concentrated the filtrate under reduced pressure at 40°C, recovered dichloromethane, dissolved the residue with 10ml of acetone, controlled the temperature at 35°C, and slowly added 30ml of water until the solid precipitated. °C, filter with suction, and dry the filter cake in vacuum at 45 °C to obtain 4.5 g of white solid I. The yield is 45%, and the content is 98.4%.
Embodiment 3
[0025] In a 250ml reaction flask, add 10g (13.4mmol) of clarithromycin oximide and 200ml of acetone, stir to dissolve, add 6.64g (40mmol) of potassium iodide, 1.6g (40mmol) of sodium hydroxide, react at 20°C for 6 hours, and the reaction ends , filter with suction, concentrate the filtrate under reduced pressure at 40°C, recover acetone, dissolve the residue with 10ml of acetone, control the temperature at 35°C, slowly add 30ml of water dropwise until the solid precipitates, keep it warm for 1 hour, lower it to 8°C, and filter with suction. The filter cake was vacuum-dried at 45°C to obtain 2.8 g of white solid I. The yield is 28%, and the content is 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com