Nucleic acid substance carrier containing degradable imine linkage as well as preparation method and application thereof
An imine bond and nucleic acid technology, which can be applied to other methods of inserting foreign genetic materials, genetic material components, medical preparations of non-effective ingredients, etc., can solve the problems of reduced transfection efficiency, escape of nucleic acid substances, slow degradation rate, etc. , to achieve the effect of simple vector structure, low cytotoxicity and high transfection activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
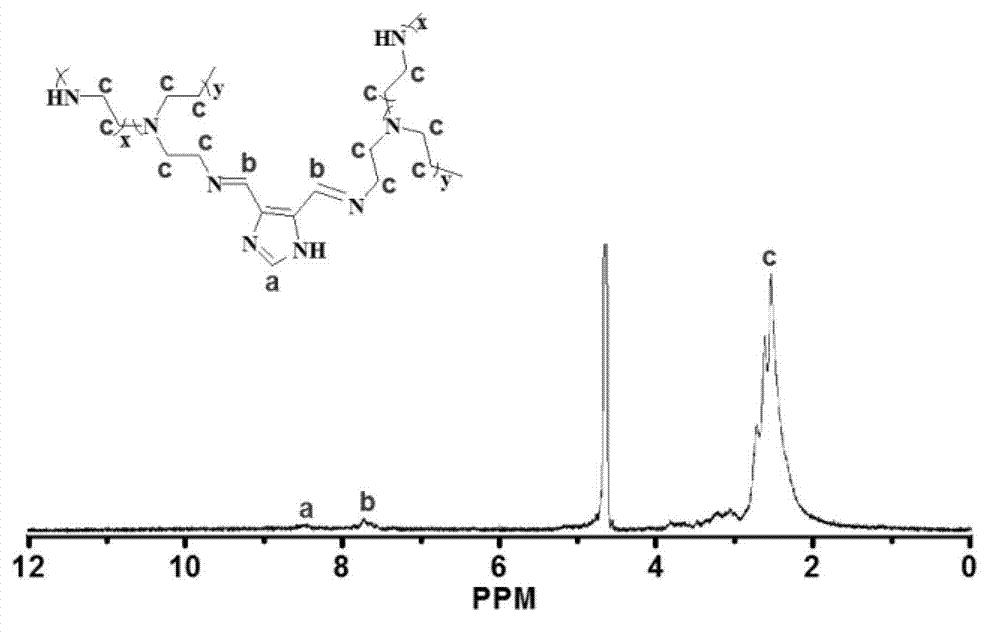
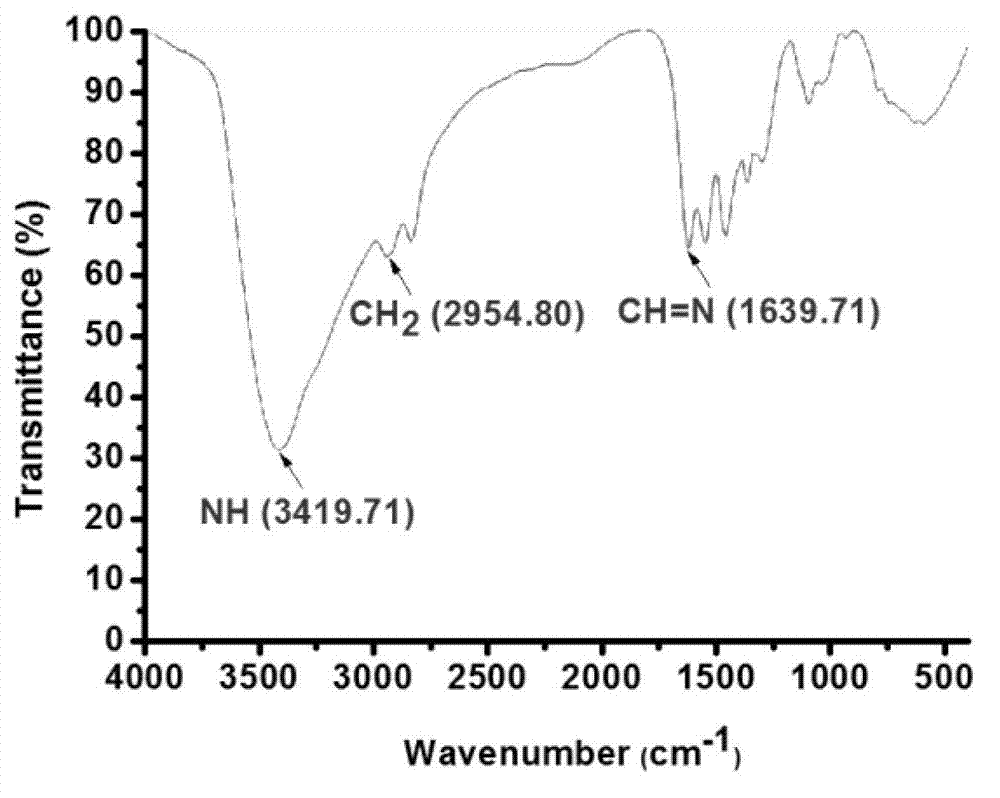
[0049] Preparation of carrier (PPI) containing degradable imine bond nucleic acid material
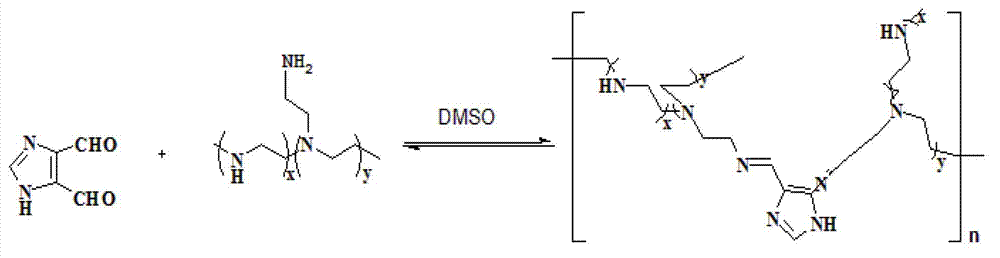
[0050] Synthetic route such as figure 1 Shown. It includes the following steps:
[0051] (1) The reaction solvent is anhydrous ethanol. After adding anhydrous magnesium sulfate to the anhydrous ethanol, let it stand for 48 hours, and then distill to obtain fresh anhydrous ethanol for use; the whole reaction is carried out in an anhydrous, oxygen-free (high-purity nitrogen protection) environment , Take the molar ratio of small molecule PEI (Mw=800Da) to imidazole dialdehyde 1:2.5, dissolve small molecule PEI and imidazole dialdehyde with absolute ethanol, and make 20mL and 50mL solutions respectively;
[0052] (2) Add the imidazole dialdehyde solution to the small molecule PEI solution drop by drop with a constant pressure dropping funnel, and stir the reaction system at room temperature to allow condensation reaction to occur for 24 hours;
[0053] (3) After the reaction is stopped, first u...
Embodiment 2
[0067] Preparation of PPI and pDNA complex
[0068] Weigh a certain amount of polymer PPI and dissolve it in ultrapure water with a concentration of 2.0mg / mL and filter it with a 0.45μm microporous membrane for use; draw a certain amount of pDNA solution and dilute it with ultrapure water to a 20μg / mL pDNA stock solution . When preparing the PPI and pDNA complex, dilute the PPI solution to the corresponding concentration according to the set mass ratio of a series of PPI and pDNA, and then quickly add it to the same volume and constant concentration of pDNA solution (the final concentration of pDNA is 2μg / mL ), and finally, gently pipet, mix evenly, and incubate at room temperature for 20-30 minutes to obtain a series of complex solutions with different mass ratios for further physical and chemical characterization.
Embodiment 3
[0070] Agarose gel electrophoresis of PPI and pDNA complex
[0071] Weigh 1.0g of agarose, add 100mL of 1×TAE buffer, heat it in a microwave oven to dissolve, wait until the temperature drops to 65℃, add 4μL of ethidium bromide (EB) to prepare 1.0% agarose solution (containing 0.5μg / mL Ethidium bromide), pour it into the glue making tank, insert the sample comb, and place it at room temperature for 0.5-1 hour to solidify into glue. Then, pull out the sample comb, add TAE buffer to the electrophoresis tank to slightly cover the gel, and wait for the sample to be loaded. Then, according to the preparation method of the compound, compound solutions with different mass ratios are prepared, and the compound mass ratios are 0, 0.5, 1, 3, 5, 10, 20, 30, 50 in sequence. Marker chooses DS TM 5000 (100-5000bp), loading 5μL; 6× loading buffer (bromophenol blue-glycerol indicator, containing 0.25% bromophenol blue, 40% glycerol) 1μL mixed with 5μL of complex solution, loading 6μL. Electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com