Synthesis of Amphiphilic β-Cyclodextrin Star Polymer and Its Micellization Application
An amphiphilic polymer and star polymer technology, which is applied in the fields of polymer chemistry and pharmaceutical preparations, can solve the problems of affecting drug efficacy and absolute bioavailability, so as to improve drug loading performance and improve bioavailability. , No toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
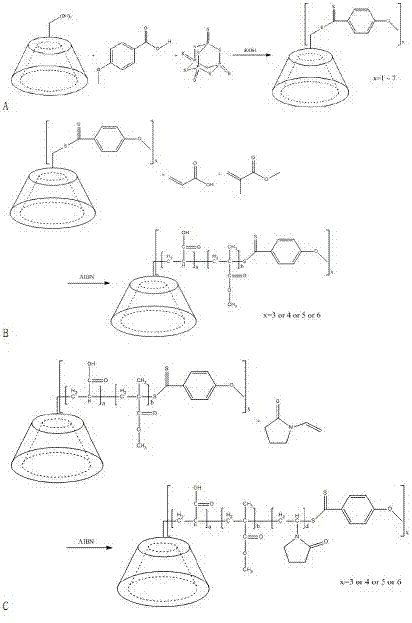
[0068] Material β-CD-[P(AA-co-MMA)-b-PNVP] 6 (PAA:PMMA:PNVP=10:1:12) specific synthesis steps
[0069] Dissolve 5.675 g β-CD, 4.566 g MBA, 0.421 g KOH, and 6.668 g P in 80 mL of 1,4-dioxane 2 S 5 , and then reflux at 120°C for 30h. After the reaction, let cool and filter, the obtained solid was washed with acetone-water (1:1, v / v) and filtered three times, and vacuum-dried at 50°C to obtain hexa-substituted dithiobenzoate β-CD (CTA4) .
[0070] Add 0.4g of CTA4 reagent to the dry reaction eggplant bottle that has been deaired by argon, add 25mL of ethanol-water (1.5:1) mixed solvent to dissolve; then add 10.38mL of AA, 1.07mL of MMA, 0.016g of AIBN and then carry out circulation pumping Exhaust - fill in argon to remove oxygen, then seal the reaction at 70°C for 18 hours; after the reaction, take out the reaction bottle and cool it, the reaction mixture is precipitated with ethyl acetate, and the resulting precipitate is vacuum-dried at 45°C to obtain β-CD- [P(AA-co...
Embodiment example 2
[0074] Material β-CD-[P(AA-co-MMA)-b-PNVP] 4 (PAA:PMMA:PNVP=7:1:8) specific synthesis steps
[0075] Dissolve 5.675 g β-CD, 2.283 g MBA, 0.421 g KOH, and 3.334 g P in 80 mL of 1,4-dioxane 2 S 5 , and then reflux at 120°C for 30h. After the reaction, let cool and filter, the obtained solid was washed with acetone-water (1:1, v / v) and filtered three times, and vacuum-dried at 50°C to obtain tetrasubstituted dithiobenzoate β-CD (CTA2) .
[0076] Add 0.4g of CTA2 reagent to the dry reaction eggplant bottle that has been deaired by argon, add 25mL of ethanol-water (1.5:1) mixed solvent to dissolve; then add 8.10mL of AA, 1.14mL of MMA, 0.016g of AIBN and then carry out circulation pumping Exhaust - fill in argon to remove oxygen, then seal the reaction at 70°C for 18 hours; after the reaction, take out the reaction bottle and cool it, the reaction mixture is precipitated with ethyl acetate, and the resulting precipitate is vacuum-dried at 45°C to obtain β-CD- [P(AA-co-MM...
Embodiment example 3
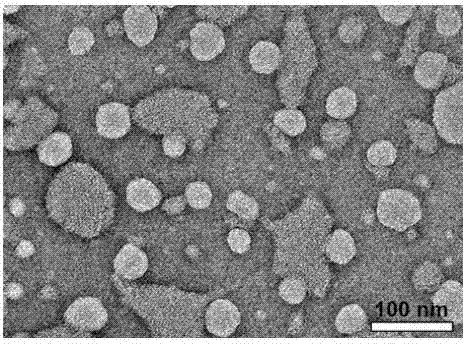
[0079] Implementation Case 3 The process of preparing micelles by thin film hydration method
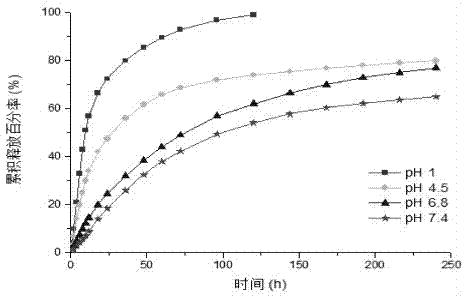
[0080] Accurately weigh 40 mg of the product prepared in Example 2 (β-CD-[P(AA-co-MMA)-b-PNVP] 4(PAA:PMMA:PNVP=7:1:8)) and 20mg of vinpocetine were placed in a 250mL dry round bottom flask with magnetic stirring; add 50mL of methanol and stir to dissolve the polymer and the drug and disperse evenly in the organic solvent ;Place the flask in a 40°C water bath, remove methanol by rotary evaporation under reduced pressure, so that the solute forms a film and adheres to the wall of the flask; transfer to a 60°C water bath and keep warm for 5min to melt the solid skeleton film; add 10mL of 60°C deionized water , stirred for 2h (1500r / min) to hydrate into micelles; quenched to room temperature, and filtered out the uncoated drug with a 0.22μm syringe filter to obtain a drug-loaded micellar solution with a drug loading of 3.52%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com