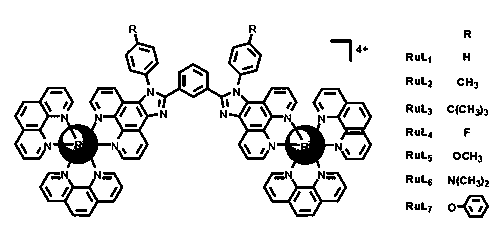
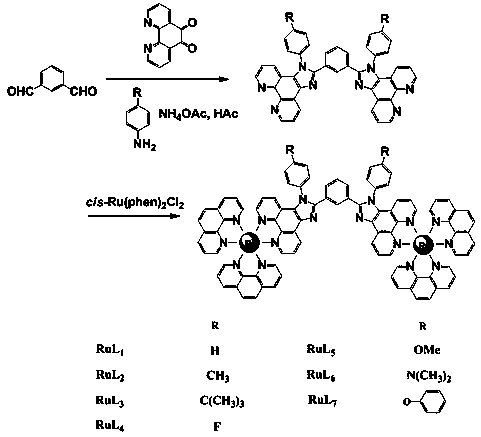
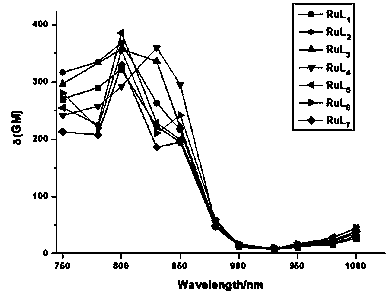
Dinuclear ruthenium complex as well as preparation method and application thereof as living cell fluorescent dye
A dual-nuclear ruthenium complex and fluorescent dye technology, which is applied in the field of cell imaging reagents, can solve the problems of unfavorable differentiation of endogenous fluorescent dyes, self-quenching, unfavorable imaging stability, low photostability, etc., and achieves good cell membrane permeability and cell permeability. Strong ability, simple structure and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Binuclear ruthenium complex RuL 1 preparation of
[0043] (1) Using 1,10-phenanthroline-5,6-dione, ammonium acetate, m-phenylacetaldehyde, aniline, and glacial acetic acid as raw materials, mix and heat to reflux for 24 hours under the protection of argon. After the mixture was cooled to room temperature, water was added, neutralized with 25% ammonia water, filtered with suction to obtain a yellow precipitate, washed with water and ether, and the product was recrystallized with methanol to obtain a yellow powder 1,3-bis(1-phenyl-1H-imidazo[4 ,5-f][1,10]phenanthrolin-2-yl)benzene (ie L 1 ). Yield: 85%. Anal. Calcd for (elemental analysis) C 44 h 26 N 8 : C, 79.26; H, 3.93; N, 16.81. Found: C, 79.12; H, 3.45; N, 24.39%. 1 H NMR (500 MHz, DMSO-d 6 ) ppm: 9.11 (d, J = 5.0 Hz, 2H), 9.03 (d, J = 5.0 Hz, 2H), 8.97 (d, J = 5.0 Hz, 2H), 8.17 (s, 1H), 7.93 (m, 2H), 7.76 (m, 8H), 7.66 (m, 2H), 7.56 (d, J = 5.0 Hz, 2H), 7.48 (d, J = 5.0 Hz, 2H), 7.36 (t, J ...
Embodiment 2
[0045] Example 2 Binuclear ruthenium complex RuL 2 preparation of
[0046] Preparation steps are the same as RuL in embodiment 1 1 The difference is that the aniline in step (1) is replaced by p-methylaniline, and the remaining steps and operations remain unchanged.
[0047] The intermediate product 1,3-bis(1-p-tolyl-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzene (ie L 2 ) Yield: 83%. Anal. Calcd for C 46 h 30 N 8 : C, 79.52; H, 4.35; N, 16.13. Found: C, 79.48; H, 4.42; N, 16.10%. 1 H NMR (500 MHz, DMSO-d 6 ) ppm: 9.10 (d, J = 5.0 Hz, 2H), 9.01 (d, J = 5.0 Hz, 2H), 8.96 (d, J = 5.0 Hz, 2H), 8.25 (s, 1H), 7.91 (m, 2H), 7.63 (d, J =5.0 Hz, 4H), 7.53 (d, J =5.0 Hz, 4H), 7.51 (m, 2H), 7.47 (d, J = 5.0 Hz, 2H), 7.40 (d, J = 5.0 Hz, 2H), 7.30 (t, J 1 =J 2 =5.0 Hz, 1H) , 2.52 (s, 6H). FAB-MS: m / z = 695 [M+1].
[0048] Final product [(phen) 2 Ru(L 2 )Ru(phen) 2 ] (ClO 4 ) 4 (i.e. RuL 2 ) Yield: 66%. Anal. Calcd for C 94 h 62 Cl 4 N 16 o 16 Ru 2 : C, ...
Embodiment 3
[0049] Example 3 Binuclear ruthenium complex RuL 3 preparation of
[0050] Preparation steps are the same as RuL in embodiment 1 1 The difference is that the aniline in step (1) is replaced by 4-tert-butylaniline, and the remaining steps and operations remain unchanged.
[0051] The intermediate product 1,3-bis(1-(4-tert-butylphenyl)-1H-imidazo[4,5-f][1,10]phenanthrolin- 2-yl)benzene (ie L 3 ) Yield: 80%. Anal. Calcd for C 52 h 42 N 8 : C, 80.18; H, 5.43; N, 14.39. Found: C, 80.09; H, 5.48; N, 14.43%. 1 H NMR (500 MHz, DMSO-d 6 ) ppm: 9.11 (d, J = 5.0 Hz, 2H), 9.03 (d, J = 5.0 Hz, 2H), 8.97 (d, J = 5.0 Hz, 2H), 8.00 (s, 1H), 7.91 (m, 2H), 7.72 (d, J =5.0 Hz, 4H), 7.62 (d, J =5.0 Hz, 2H), 7.52 (m, 2H), 7.46 (d, J = 5.0 Hz, 2H), 7.39 (d, J = 5.0 Hz, 1H), 7.33 (t, J 1 =J 2 =5.0 Hz, 1H), 7.29 (d, J = 5.0 Hz, 3H), 1.25 (s, 18H). FAB-MS: m / z = 779 [M+1].
[0052] Final product [(phen) 2 Ru(L 3 )Ru(phen) 2 ] (ClO 4 ) 4 (i.e. RuL 3 ) Yield: 65%. Anal. Calcd for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com