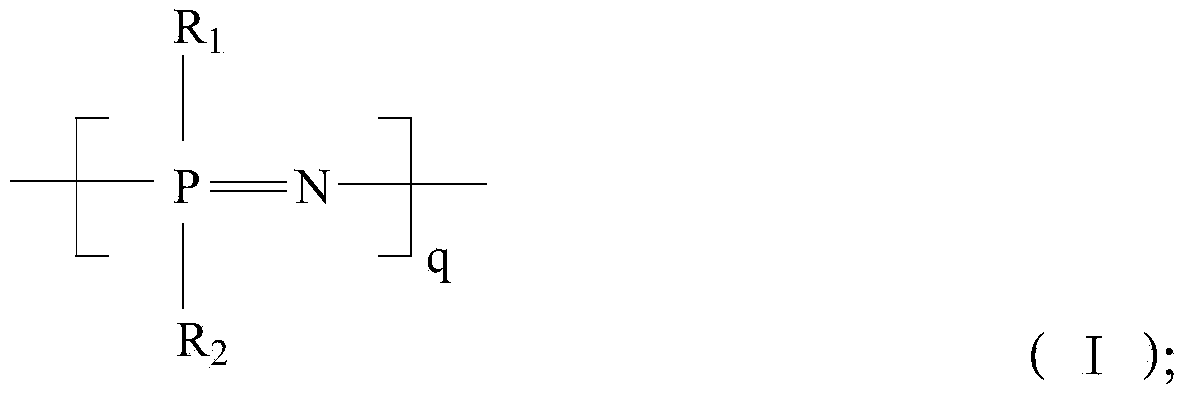Electrolyte
An electrolyte and polyphosphazene technology, applied in the electrolyte field, can solve the problems of high synthesis cost of fluorinated additives, decreased electrolyte conductivity, complex electrolyte system, etc., and achieves good flame retardancy, improved battery safety, good wettability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
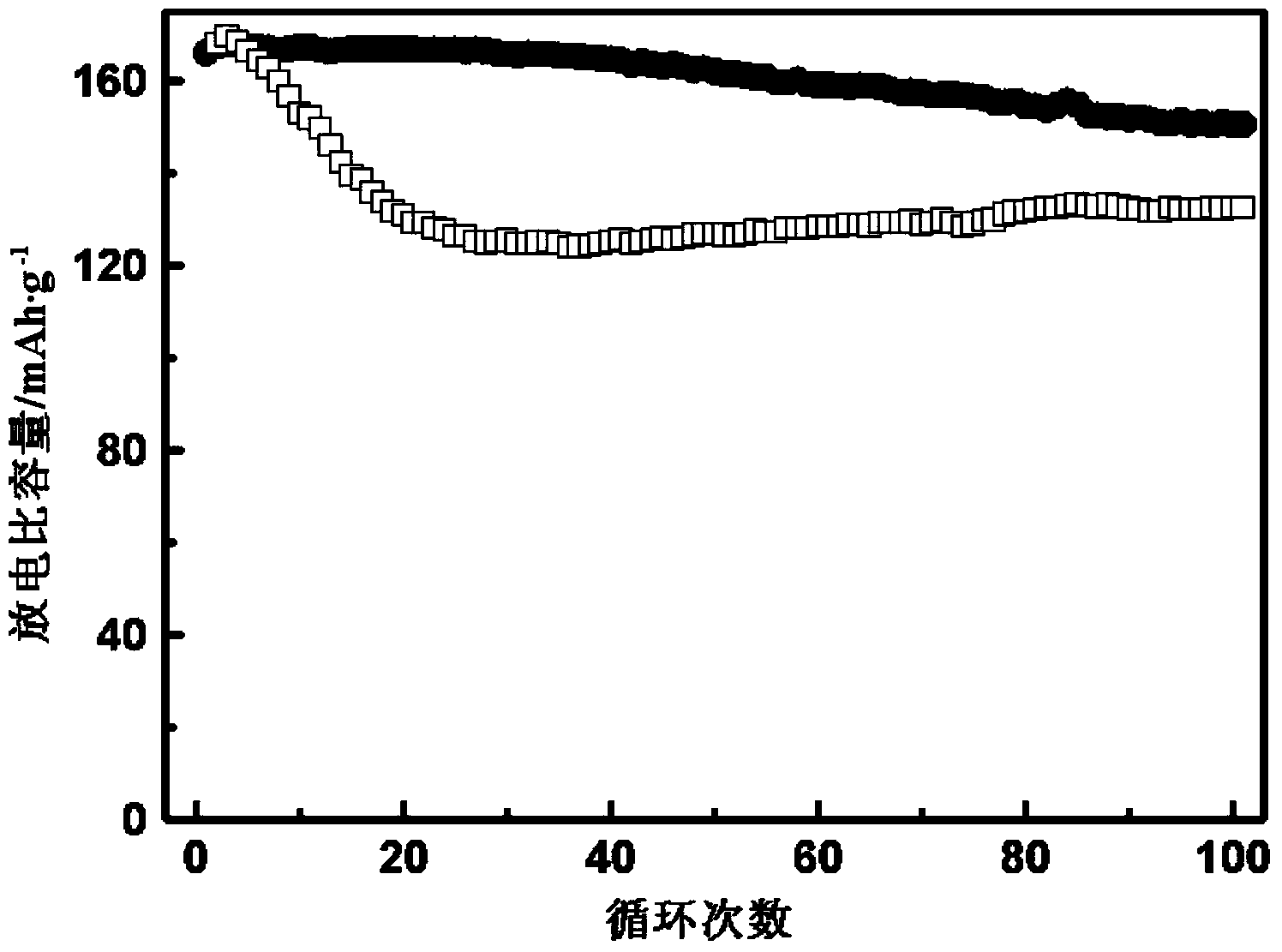
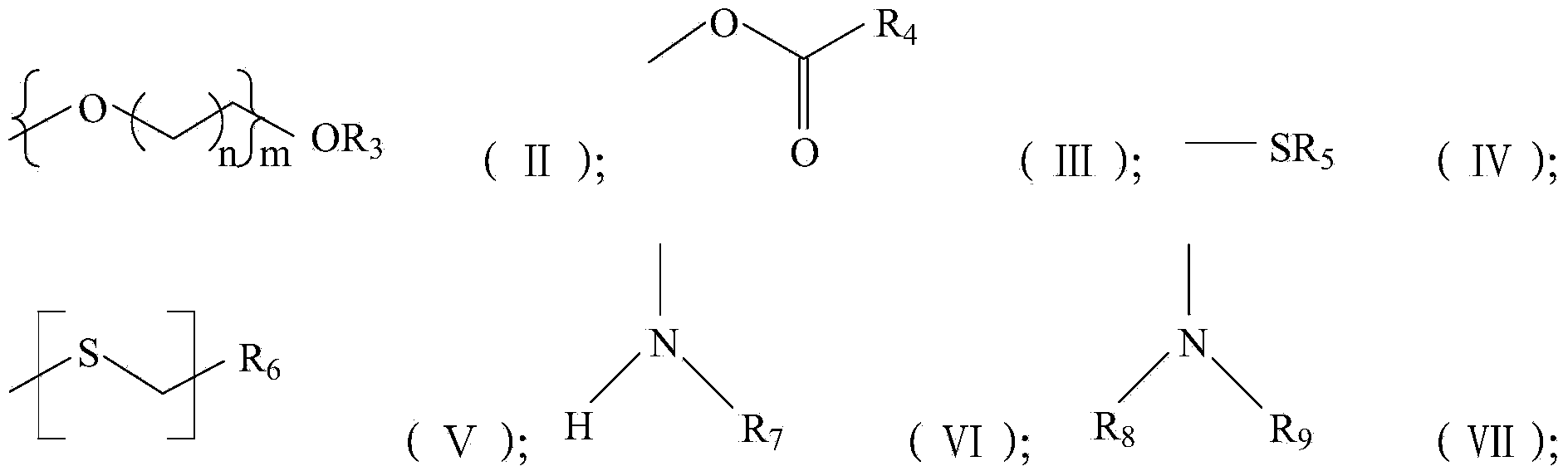
Image
Examples
Embodiment 1
[0043] Put 25g of hexachlorotrimeric phosphazene, 0.2g of sulfamic acid, 0.15g of calcium sulfate dihydrate, and 50mL of 1-chloronaphthalene into a 500mL four-necked flask equipped with a condenser tube, a drying tube, a thermometer, and mechanical stirring. Under the protection of the temperature rise to 230 ℃, constant temperature reaction for several hours, when the viscosity changes significantly, stop the reaction;
[0044] Pour the above reaction solution into a 3000mL beaker containing 1000mL n-heptane, stir until a white polymer precipitates at the bottom of the cup, and separate the upper solution; the polymer at the bottom is polydichlorophosphazene;
[0045] Under the protection of nitrogen, add 200mL of dried tetrahydrofuran and 34g of sodium hydride to a 1000mL dry three-necked round-bottomed flask, then slowly drop triethylene glycol monoethyl ether (189g) in tetrahydrofuran (200mL) solution, and stir at room temperature React for 2 hours. Then take 16g of polyd...
Embodiment 2
[0048] In a glove box filled with argon (moisture <<1ppm, oxygen <1ppm), take 10mL of organic mixed solution of ethylene carbonate and dimethyl carbonate, the volume ratio of ethylene carbonate and dimethyl carbonate is 3:7, Add lithium hexafluorophosphate to the organic mixed solution so that the molar concentration is 1mol / L, and finally slowly add 0.01% of the total mass of the electrolyte to the mixed solution The polybis(diethylene glycol monomethyl ether) phosphazene prepared in Example 1 (EEEP), and the lithium-ion battery electrolyte was obtained after stirring evenly.
Embodiment 3
[0050] In a glove box filled with argon (moisture <<1ppm, oxygen <1ppm), take 10mL of organic mixed solution of ethylene carbonate and dimethyl carbonate, the volume ratio of ethylene carbonate and dimethyl carbonate is 3:7, Add lithium hexafluorophosphate to the organic mixed solution to make the molar concentration 1mol / L, and finally slowly add the polybis(diethylene glycol monomethyl ether) phosphazene prepared in Example 1 of 5% of the total mass of the electrolyte to the mixed solution (EEEP), and the lithium-ion battery electrolyte was obtained after stirring evenly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com