A mutant tba-h2 of acid-resistant high-temperature beta-amylase and its application
A technology of TBA-H2 and amylase, applied in the direction of enzyme, hydrolase, glycosylase, etc., can solve the problem that β-amylase cannot take into account acid resistance and temperature resistance at the same time, so as to improve catalytic activity and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
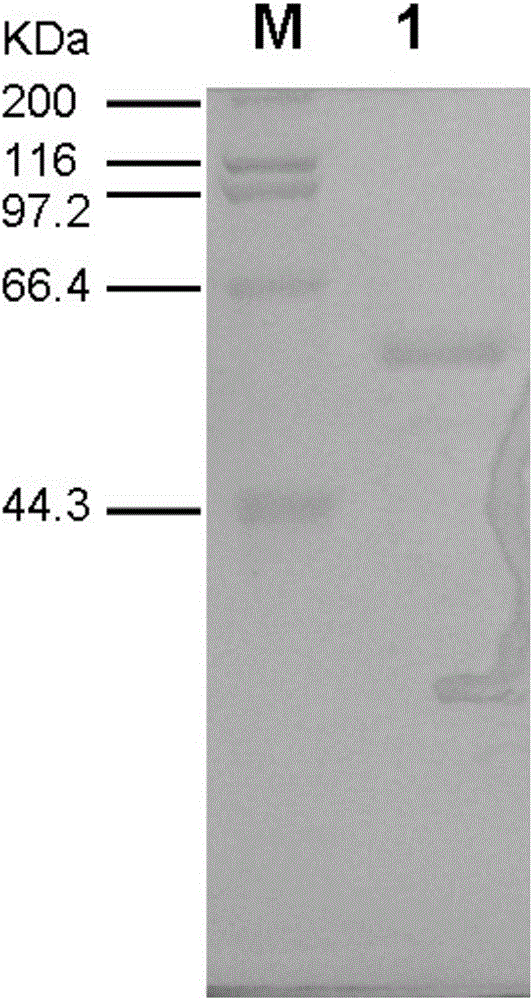
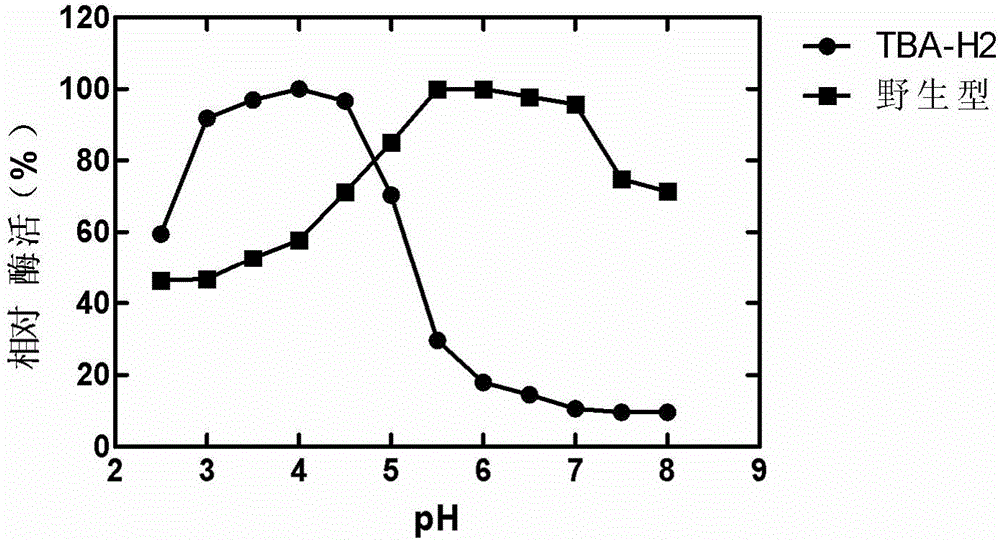
[0017] This example illustrates the method for obtaining the β-amylase mutant TBA-H2.
[0018] 1) Cloning of β-amylase gene ctba
[0019] The chromosomal DNA of Clostridium thermothiobacterium (Thermoanaerobacterium thermosulfurigenes, American Type Culture Collection No. ATCC33743) was used as the template, SEQ ID NO: 2 was used as the upstream primer (containing a NcoI restriction site) and SEQ ID NO: 3 (containing a BamHI restriction site) was used as the template. site, and introduced a 6x histidine tag coding sequence) as the downstream primer, amplifying the mature peptide coding region of β-amylase by polymerase chain reaction (PCR), corresponding to the 660-2219 fragment in the ctba gene, the resulting The length of the target product is 1605bp; the target product and the pSE380 vector plasmid were double digested with NcoI and BamHI enzymes, respectively, and after the gel was recovered, ligated with T4 DNA ligase, and transformed into E. coli XL1-Blue competent cells...
Embodiment 2
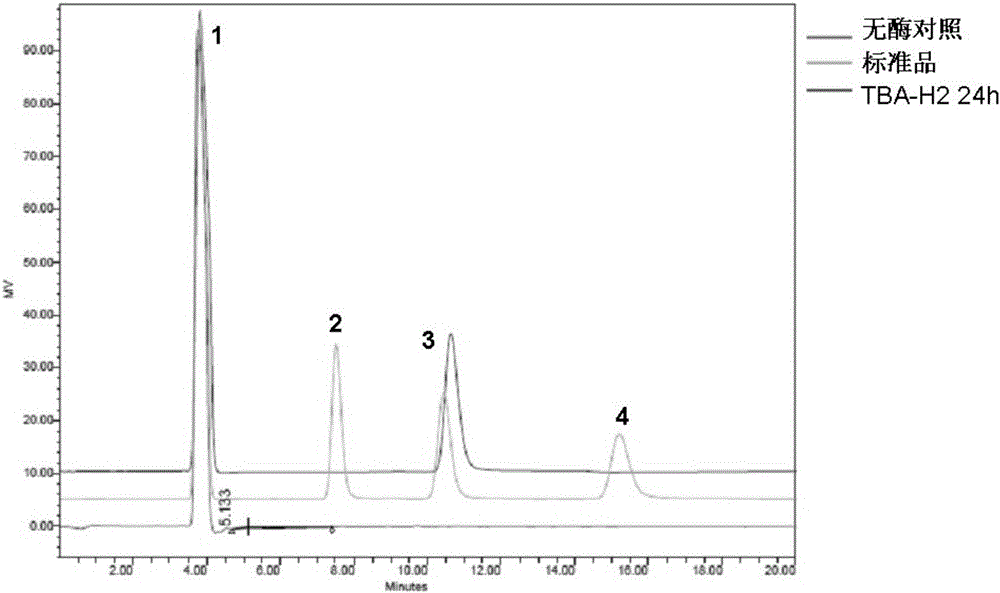
[0026] This example illustrates the application of mutant enzyme TBA-H2 in the production of high-purity maltose syrup by hydrolyzing starchy raw materials
[0027] Take 1g of potato soluble starch and add 0.05mol / LpH4.0 acetic acid buffer solution to prepare 1% (mass percentage) starch slurry, add purified enzyme solution according to the amount of 1.2mg enzyme / g dry matter starch, and then place at 60°C Warm in a water bath for 24 hours, heat-treat in a water bath at 100°C for 10 minutes, centrifuge at 12,000 r / min for 5 minutes, filter the supernatant through a 0.22 μm filter membrane, and take 20 μL of the filtrate to pass through an amino column to detect the enzymatic hydrolysis product by high performance liquid chromatography (HPLC). The detection conditions of the amino column were RI temperature of 50°C, flow rate of 1mL / min, and mobile phase of acetonitrile:water (70:30). Amino column detection results such as image 3 shown. It can be seen that under acidic condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com