Method for determining content of bipolymer impurities in faropenem sodium
A technology of faropenem sodium and a determination method, applied in the field of drug analysis, can solve the problem of not involving the quantitative detection of faropenem dimer impurities and the like, and achieve the effects of good correlation, strong specificity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
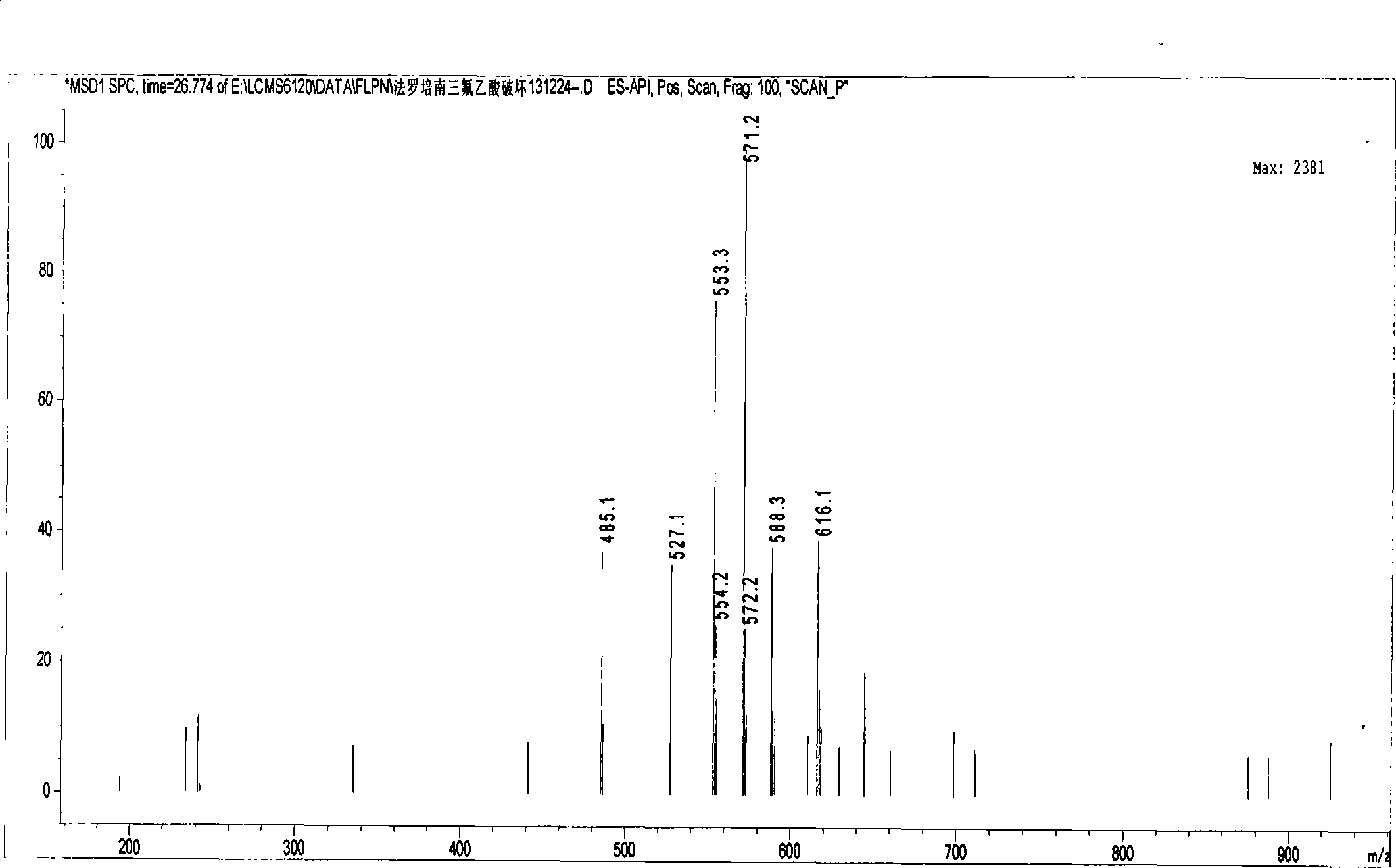
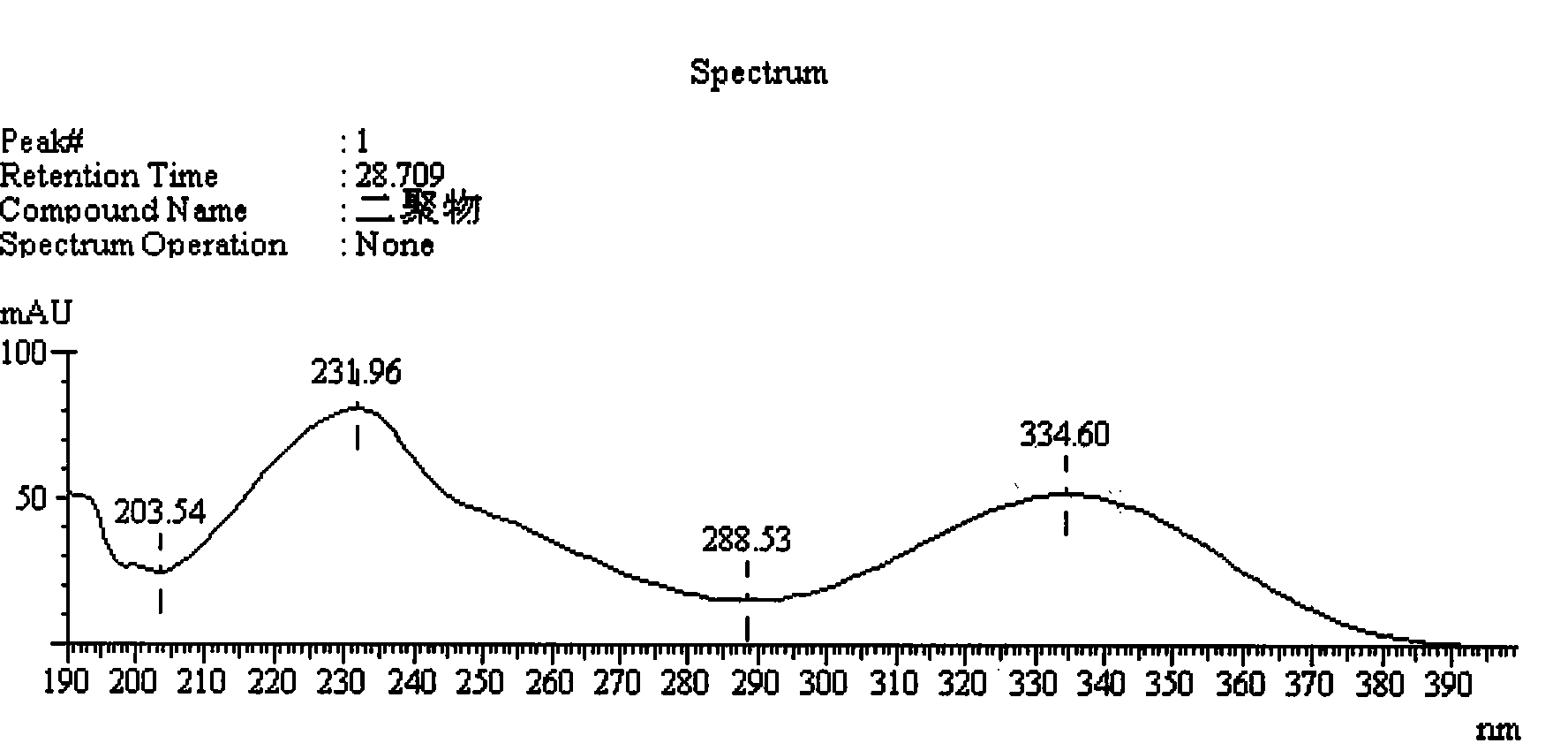
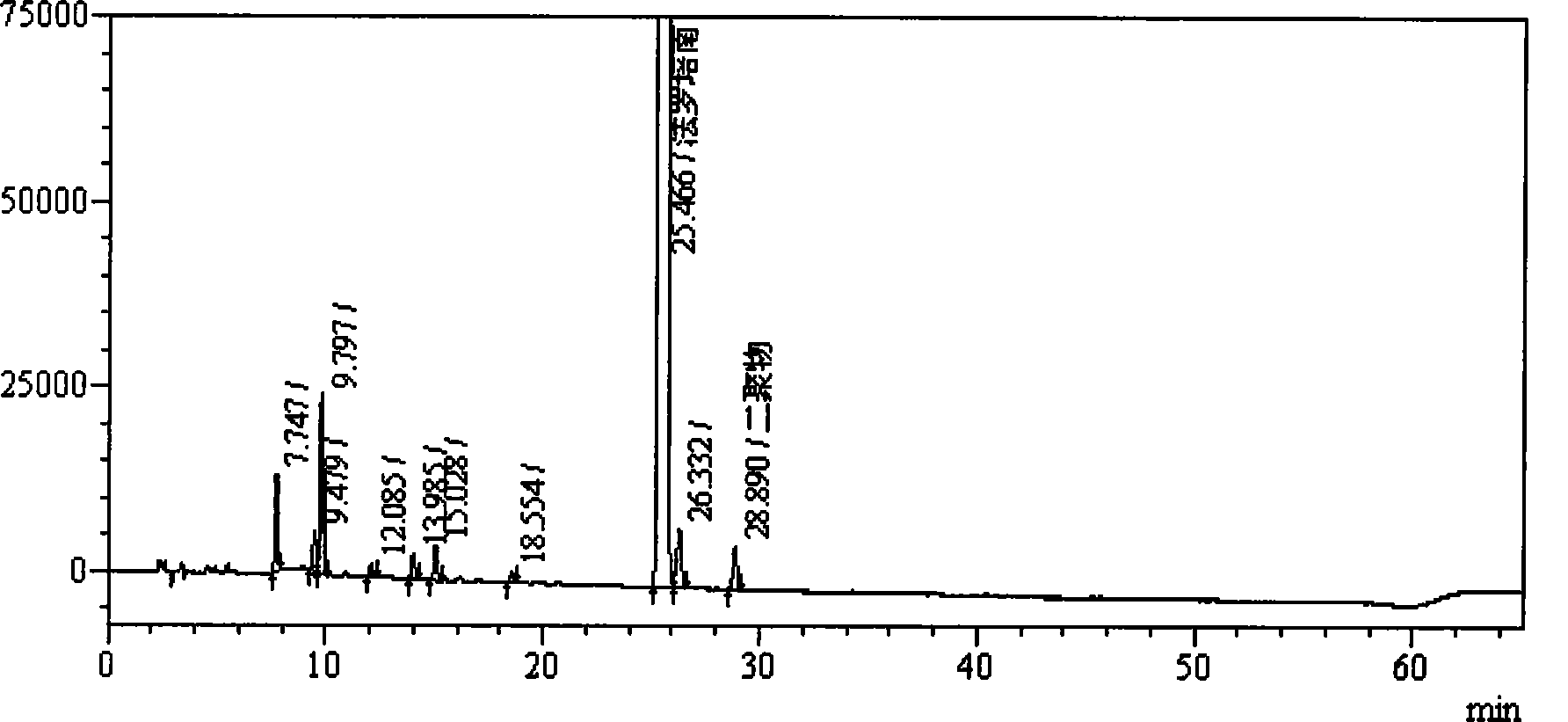
[0034]Example 1 Preparation of supply solution containing faropenem dimer and confirmation of the dimer
[0035] 1. Instruments and reagents:
[0036] Instruments and reagents: Shimadzu LC-20A high performance liquid chromatography; trifluoroacetic acid, acetonitrile.
[0037] Drugs: Faropenem Sodium DiSesquihydrate, from United Laboratories Co., Ltd.,
[0038] Batch number: 4201304001.
[0039] 2. Experimental method:
[0040] 2.1 Preparation of faropenem sample solution containing dimer: Take about 50 mg of faropenem sodium di-sesquihydrate sample (about 40 mg based on anhydrous faropenem), put it in a 20ml measuring bottle, add trifluorotrifluoroethylene with a volume concentration of 0.05% Aqueous acetic acid was dissolved and diluted to volume, and left overnight at room temperature.
[0041] 2.2 Chromatographic conditions: Shimadzu LC-20A high performance liquid chromatograph is used; specific chromatographic conditions are: chromatographic column: octadecylsilane bo...
Embodiment 2
[0055] Example 2 Detection of Dimer Content in Faropenem Raw Materials
[0056] 1. Instruments and reagents:
[0057] Instruments and reagents: Shimadzu LC-20A high performance liquid chromatography; trifluoroacetic acid, acetonitrile.
[0058] Drugs: Faropenem Sodium DiSesquihydrate, from United Laboratories Co., Ltd.,
[0059] Batch number: 4201304001.
[0060] 2. Detection method
[0061] 2.1 Preparation of faropenem samples containing dimers: take about 1 g of faropenem sodium sesquihydrate samples, place them flat in a flat weighing bottle, add 0.1% trifluoroacetic acid aqueous solution dropwise to moisten , dried in an oven at 60°C, after the dried sample was finely ground, it was then wetted by dripping an aqueous solution of trifluoroacetic acid with a volume concentration of 0.1%, and then dried in an oven at 60°C, and the operation was repeated until the resulting dimer The amount of the substance is obvious enough (the peak area percentage is greater than 0.5%),...
Embodiment 3
[0073] Example 3 Detection of dimers in conventional faropenem raw materials
[0074] 1. Instruments and reagents:
[0075] Instruments and reagents: Shimadzu LC-20A high performance liquid chromatography; trifluoroacetic acid, acetonitrile.
[0076] Drugs: Faropenem Sodium Sesquihydrate Raw Material, from United Laboratories Co., Ltd.
[0077] Batch number: 4201304001.
[0078] 2. Detection method:
[0079] 2.1 Accelerated drug stability test: The raw material of faropenem sodium bis hemihydrate was used for the accelerated stability test. The experimental conditions were: temperature 40°C±2°C, relative humidity 75%±5%, and storage for 6 months.
[0080] 2.2 Preparation of the test solution: Take about 25 mg of the above-mentioned ropenem sodium di-sesquihydrate sample (about 20 mg based on anhydrous faropenem) that has undergone the accelerated stability test, put it in a 10ml measuring bottle, add water to dissolve and dilute to the mark, Shake well, as the test solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com