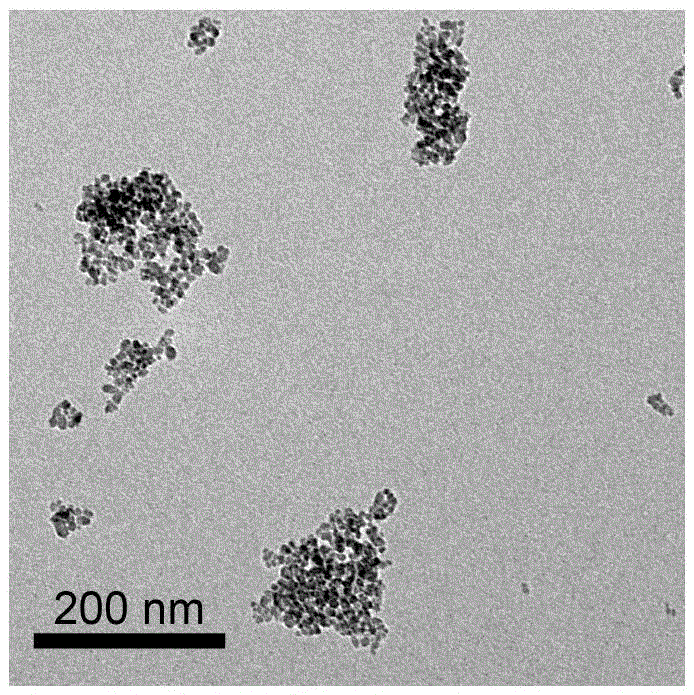
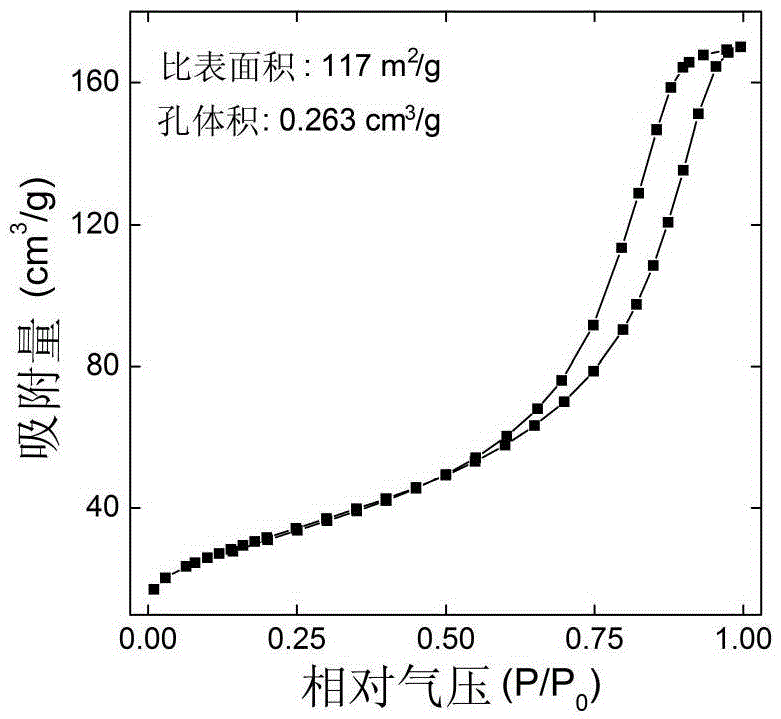
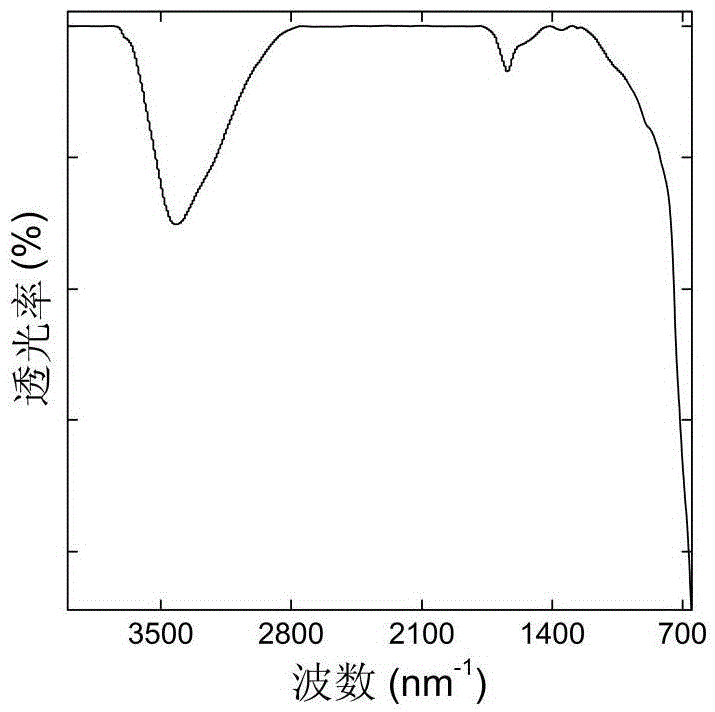
Surface-modified nanometer ferric oxide Fenton catalyst and preparation method thereof
A technology of surface modification nanometer and iron tetroxide, applied in the direction of oxidized water/sewage treatment, etc., can solve the problems of easily hindering the diffusion of pollutants and hydrogen peroxide, inability to carry out the Fenton reaction, and high production cost, and is suitable for large-scale preparation. , good catalytic efficiency, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] First take by weighing ferric sulfate and ferrous sulfate as 2.0 according to the mass ratio of ferric salt and ferrous salt, add to deionized water, then add Span 20 with a mass ratio of 3:1 to the theoretical output of ferric oxide and mix Stir to make it fully dissolved to obtain a mixed solution of iron ions; place the mixed solution of iron ions in an ultrasonic generator, and add a precipitant solution prepared by sodium citrate and potassium hydroxide dropwise while ultrasonically oscillating until the pH of the system reaches 12, of which lemon The concentration of sodium hydroxide is 0.05g / mL, the concentration of potassium hydroxide is 2mol / L, and the ultrasonic vibration is continued for 10 minutes, and then magnetic separation is carried out. The product is washed with water and ethanol in turn, and dried to obtain nanometer iron tetraoxide powder; Iron powder is added to the deionized water / isopropanol mixture with a mass ratio of 1:1, and ultrasonically osc...
Embodiment 2
[0041] First weigh ferric chloride and ferrous chloride by ferric salt and ferric salt mass ratio 1.5, add deionized water, then add Tween with a mass ratio of 5:1 with the theoretical output of ferric oxide. 80 and stir to make it fully dissolved to obtain a mixed solution of iron ions; place the mixed solution of iron ions in an ultrasonic generator, and add dropwise a precipitant solution prepared by potassium citrate and sodium hydroxide until the pH of the system reaches 11 while ultrasonically vibrating. Wherein the concentration of potassium citrate is 0.1g / mL, the concentration of sodium hydroxide is 1mol / L, and the ultrasonic vibration is continued for 10min, and then magnetic separation is carried out, and the product is washed with water and ethanol successively, and dried to obtain nanometer ferric oxide powder; Add ferric oxide powder into the deionized water / n-hexanol mixture with a mass ratio of 2:1, and ultrasonically vibrate to obtain a uniform dispersion (the ...
Embodiment 3
[0044] First weigh ferric nitrate and ferrous nitrate according to the mass ratio of ferric salt and ferrous salt as 2.5, add them into deionized water, and then add nonylphenol polymer with a mass ratio of 10:1 to the theoretical yield of ferric oxide. Oxyethylene ether and stir to make it fully dissolved to obtain a mixed solution of iron ions; place the mixed solution of iron ions in an ultrasonic generator, and add dropwise a precipitant solution prepared by sodium citrate and ammonia water while ultrasonically vibrating until the pH of the system reaches 11, Wherein the concentration of potassium citrate is 0.08g / mL, the concentration of ammonia water is 5mol / L, then continue ultrasonic vibration for 20min, then carry out magnetic separation, the product is washed with water and ethanol successively, and dried to obtain nano iron tetraoxide powder; Add the ferric iron powder into the deionized water / glycerol mixed solution with a mass ratio of 1:10, and obtain a uniform di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com