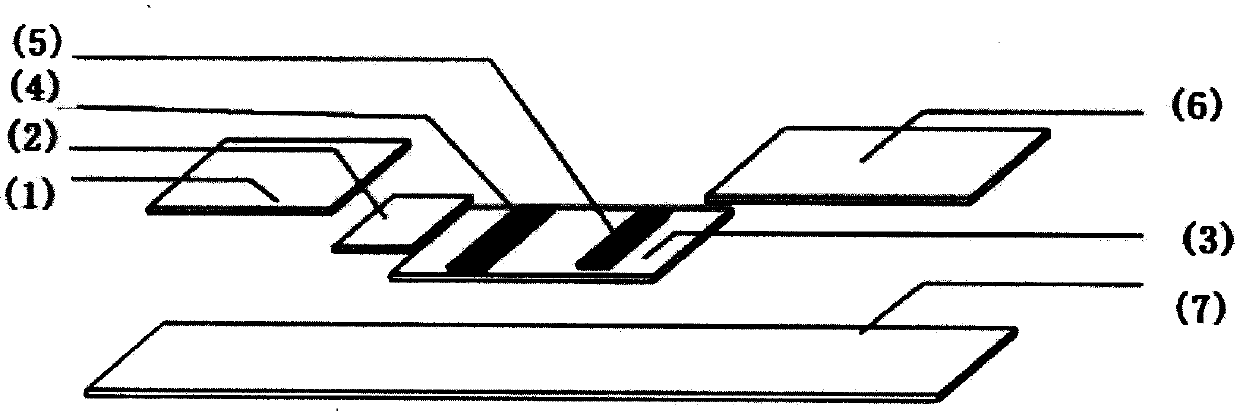
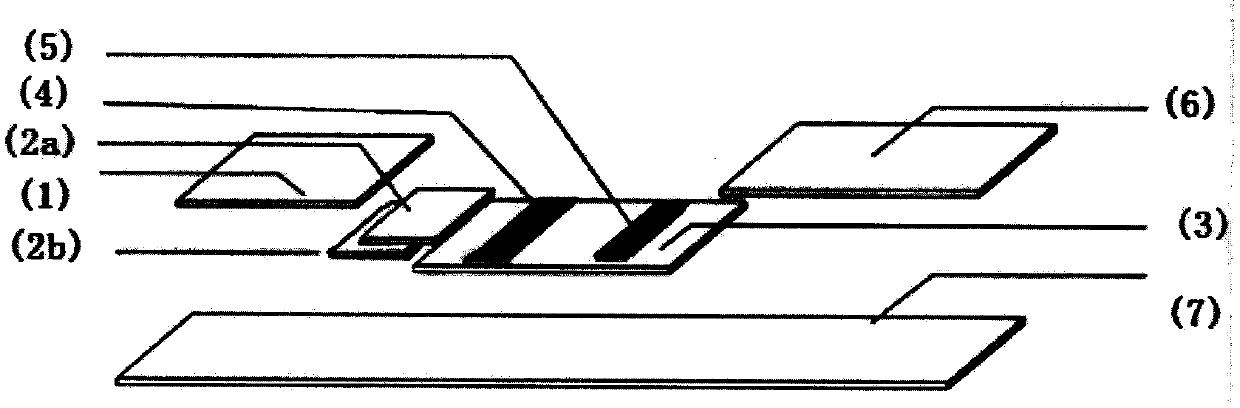
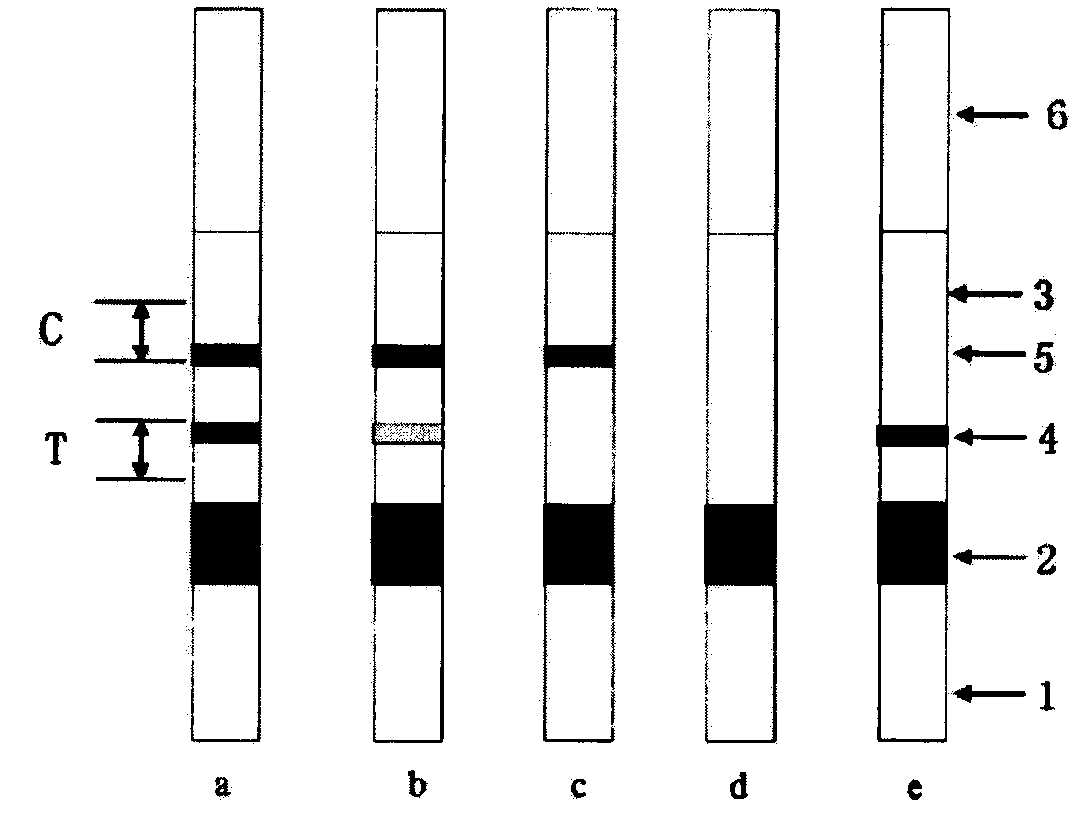
Anti-CBir1 antibody detection test paper preparation method and purpose thereof
An antibody and detection strip technology is applied in the field of preparation of immunochromatographic detection test paper to achieve the effects of high detection performance, high sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Preparation of CBir1 antigenic protein
[0048] Antigen protein preparation method 1: Recombinant protein
[0049] 1) Reagent
[0050] Escherichia coli host bacteria DH5α, BL21(DE3), cloning vector pCR2.1T-vector, expression plasmid pET28a(+), DNA polymerase rTaq, T4 DNA ligase, DNA polymerase rTaq, LA Taq and restriction enzyme BamH I , Hind III and EcoR I, BamH I, DL2000 DNA Marker, T4 DNA ligase, low molecular weight standard protein, DNA gel recovery kit, IPTG, etc.
[0051] 2) Instrument
[0052] Ordinary shaker SCS-24; water-proof constant temperature electric heating incubator; Biophotometer spectrophotometer, desktop refrigerated centrifuge Centrifuge 5810R, desktop centrifuge MiniSpin; high-speed refrigerated centrifuge; protein electrophoresis and gel imaging system; PCR instrument; ultrasound Lysis instrument; constant temperature metal bath; HIS protein purification column, etc.
[0053] 3) Experimental method
[0054] a. Vector construction:...
Embodiment 2
[0062] Example 2 Antibody Preparation
[0063] Anti-human IgG monoclonal and polyclonal antibodies coated with antibodies for the conjugate pad and detection zone, as well as monoclonal and polyclonal antibodies from other animal sources for the quality control zone and conjugate pad, can be obtained by immunizing animals.
[0064] 1. The specific operation method of monoclonal antibody preparation is as follows:
[0065] The injection solution is obtained by mixing 0.05mg-5mg of immune preparations (respectively for goats, mice, rabbits, horses and guinea pigs) with 1 volume of Freund's complete adjuvant, and injects the injection solution into multiple parts of the animal skin;
[0066] One month later, the Freund's complete adjuvant mixture was subcutaneously injected into multiple parts of the animals to boost the immunization. After 7 to 14 days, the animals were bled, and the antiserum titers were measured.
[0067] Animals were boosted until titers plateaued. Hybrido...
Embodiment 3
[0073] Preparation of each component of the gold standard test paper of embodiment 3
[0074] 1. Preparation of colloidal gold solution
[0075] 0.01% (w / v) of HAuCl 4 Heat the solution to boiling, quickly add every 100mL HAuCl 4 Add an appropriate amount of reducing agent solution to the solution, and the color changes from blue, then light blue, to blue, then red after heating, and transparent orange-red after boiling for 7-10 minutes. Then filter with ultrafiltration or microporous membrane (0.45μM) to remove polymers and other impurities that may be mixed therein. The prepared colloidal gold should be pure, translucent, free of sediment and floating matter, and discard when oily matter and a large amount of black granular precipitate impurities appear on the liquid surface.
[0076] The reducing agent used therein can be trisodium citrate, tannic acid-trisodium citrate, white phosphorus, preferably trisodium citrate, more preferably 1% (w / v) trisodium citrate.
[0077]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com