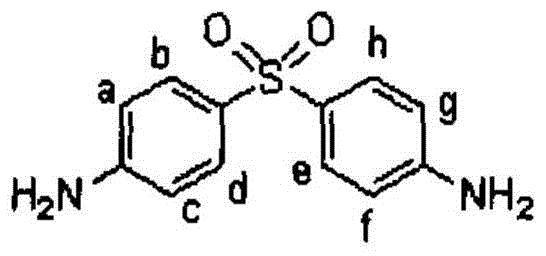
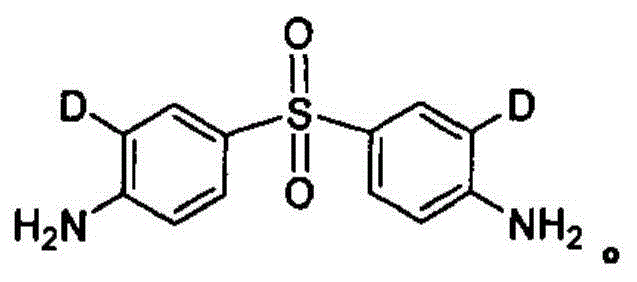
Synthetic method of stable isotope labeled aminophenylsulfone
A technology of stable isotope and synthesis method, which is applied in the synthesis field of stable isotope-labeled dapsone, can solve the problems of long reaction steps, high cost of raw material aniline and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] A stable isotope labeled dapsone-D 2 The preparation method comprises the following steps:
[0164] 1. Stable isotope labeling 4,4'-dinitrodiphenylsulfide-D 2 preparation of
[0165] Add 10.3g p-nitrobromobenzene-D to a 250mL three-necked flask 1 , add 20mL of water, stir and disperse, add 7g of sodium sulfide, add dropwise 50mL of 20% potassium carbonate aqueous solution at 0°C, after the dropwise addition is completed, rise to room temperature, react for 4 hours, filter with suction and wash with water to obtain 6.5g of light yellow 4,4 '-Dinitrodiphenylsulfide-D 2 , yield 93.5%, GC detection, purity 99.5%; mass spectrometry detection, abundance 99.5atom%D.
[0166] 2. Stable isotope labeling 4,4'-dinitrodiphenylsulfone-D 2 preparation of
[0167] In a 100mL three-necked flask, add 2.78g 4,4'-dinitrodiphenylsulfide-D 2 , add 5mL deuterated acetic acid, 3g potassium permanganate, control temperature at 0°C, react for 4 hours, pour into water, filter with suction...
Embodiment 2
[0172] A stable isotope labeled dapsone-D 1 The preparation method comprises the following steps:
[0173] 1. Stable isotope labeling 4,4'-dinitrodiphenylsulfide-D 1 preparation of
[0174] Add 5.1g p-nitrobromobenzene-D to a 250mL three-necked flask 1 , 4.5 p-nitrochlorobenzene, add 30mL of methanol and stir to dissolve, then add 1g of sodium methoxide under temperature control at -5°C, add 50mL of sodium sulfide aqueous solution dropwise, after the dropwise addition, control temperature at -5°C to 5°C, react for 2 hours, pour Pour into water, filter with suction and wash with water to obtain 6.3g light yellow 4,4'-dinitrodiphenylsulfide-D 1 , yield 91.5%, GC detection, purity 99.4%; mass spectrometry detection, abundance 99.2atom%D.
[0175] 2. Stable isotope labeling 4,4'-dinitrodiphenylsulfone-D 1 preparation of
[0176] In a 100mL three-necked flask, add 13.9g 4,4'-dinitrodiphenylsulfide-D 1 , add 50mL of deuterated chloroform, stir to dissolve, add 15mL of peracet...
Embodiment 3
[0181] A stable isotope labeled dapsone-D 4 The preparation method comprises the following steps:
[0182] 1. Stable isotope labeling 4,4'-dinitrodiphenylsulfide-D 4 preparation of
[0183] Add 14.5g p-nitrofluorobenzene-D to a 250mL three-necked flask 2 , add 30 mL of ethanol and stir to dissolve, then add 3 g of sodium ethoxide under temperature control at -5 ° C, add 50 mL of sodium sulfide aqueous solution dropwise, after the dropwise addition, control the temperature at 20 ° C for 5 hours, pour into water, filter with suction and wash with water to obtain 13.5 g of light yellow 4,4'-Dinitrodiphenylsulfide-D 4 , yield 96.4%, GC detection, purity 99.2%; mass spectrometry detection, abundance 99.3atom%D.
[0184] 2. Stable isotope labeling 4,4'-dinitrodiphenylsulfone-D 4 preparation of
[0185] In a 100mL three-necked flask, add 7g of 4,4'-dinitrodiphenylsulfide-D 4 , add 50mL deuterated acetonitrile, stir to dissolve, add 15g chromium trioxide, react at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com