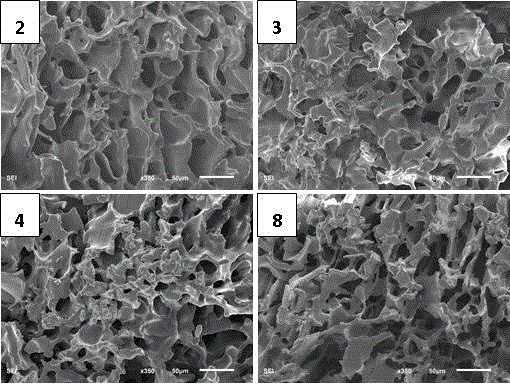
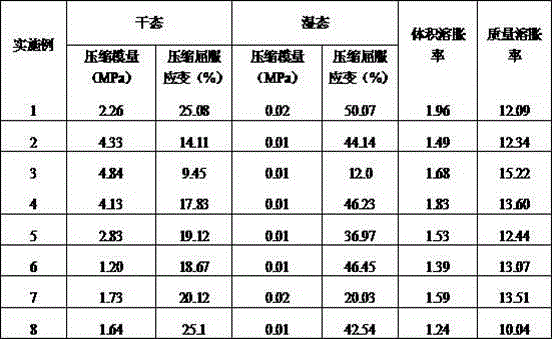
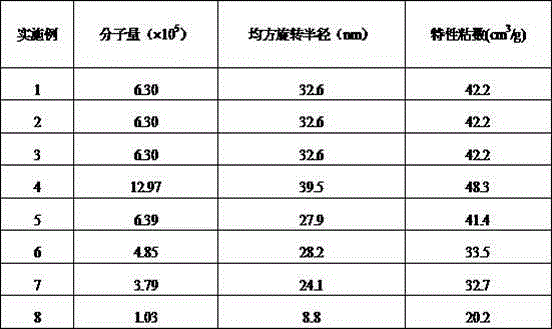
Method of preparing highly branched polysaccharide-fibroin hydrogel bracket
A polysaccharide and hydrogel technology, which is applied in the field of preparation of highly branched polysaccharide-silk fibroin hydrogel scaffolds, can solve the problem of difficult to support cell adhesion and proliferation growth, the large amount of organic raw materials diamine or hydrazine, and the inside of the scaffold and uneven surface crosslinking to achieve good controllable release behavior, improved mechanical properties, and high swelling properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] A kind of method for preparing highly branched polysaccharide-silk fibroin hydrogel support, described preparation method comprises the following steps:
[0020] a The dried sclerotia of tiger milk mushroom was crushed, and the fat was removed by Soxhlet extraction with ethyl acetate and acetone for 6 h sequentially. Both ethyl acetate and acetone were chemical pure reagents. Then soak the sclerotium of tiger milk mushroom after fat removal in physiological saline at 80 ℃ for 2 h, centrifuge; extract the residue under high pressure at 120 ℃ for 30 min, and centrifuge at 8000 rpm for 20 min to obtain the extract , after cooling the extract, centrifuge and collect the residue; the residue is centrifugally washed with deionized water and freeze-dried to obtain highly branched tiger milk mushroom polysaccharide, and can also be dried by other methods to obtain highly branched tiger milk mushroom polysaccharide, and the heavy precipitation classification method is used The o...
Embodiment 1
[0028] 0.9 g molecular weight is 4.81´10 6 The hyperbranched polysaccharides of tiger milk mushroom were dispersed in 50 mL of 20 wt% NaOH and isopropanol mixed solution, stirred in an ice-water bath for 2 h to form a uniform suspension, and 7.8 g of chloroacetic acid was dissolved in isopropanol, And slowly added dropwise to the polysaccharide suspension system, and reacted at 60 °C for 3 h. After stopping the reaction, cool to room temperature, then use 0.5 M acetic acid solution to neutralize to pH = 7, dialyze the above product with distilled water, concentrate by rotary evaporation, and freeze-dry to obtain carboxymethylated hyperbranched polysaccharide. The hyperbranched polysaccharides were dissolved in phosphate buffered saline solution with pH=7.4 to obtain a 20% solution, and 100 mg / mL of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Salt and 150 mg / mL N-hydroxysuccinimide were added to the above solution, stirred for 15 min to obtain an activated syst...
Embodiment 2
[0030] 0.9 g molecular weight is 4.81´10 6 The hyperbranched polysaccharides of tiger milk mushroom were dispersed in 50 mL of 20 wt% NaOH and isopropanol mixed solution, stirred in an ice-water bath for 2 h to form a uniform suspension, and 7.8 g of chloroacetic acid was dissolved in isopropanol, And slowly added dropwise to the polysaccharide suspension system, and reacted at 60 °C for 3 h. After stopping the reaction, cool to room temperature, then use 0.5 M acetic acid solution to neutralize to pH = 7, dialyze the above product with distilled water, concentrate by rotary evaporation, and freeze-dry to obtain carboxymethylated hyperbranched polysaccharide. The hyperbranched polysaccharides were dissolved in phosphate buffered saline solution with pH=7.4 to obtain a 40% solution, and 100 mg / mL of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Salt and 150 mg / mL N-hydroxysuccinimide were added to the above solution, stirred for 15 min to obtain an activated syst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com