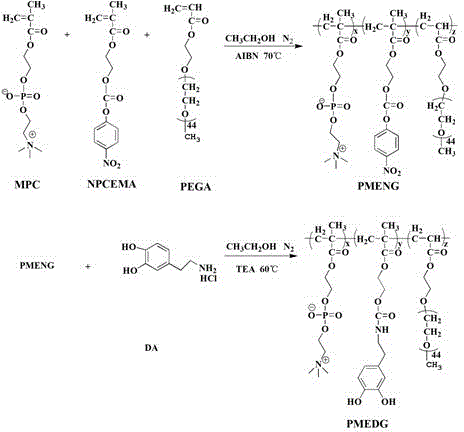
Functional polymer containing phosphorylcholine and PEG and method for forming anti-pollution coating with functional polymer
A polymer, nitrophenoxy formyl methacrylate technology, applied in coatings, biocide-containing paints, antifouling/underwater coatings, etc., to achieve simple construction methods and biocompatibility Broad, good flexibility and hydrophilic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of active ester polymerizable monomer NPCEMA
[0044]
[0045] Weigh 4.009 g of HEMA and 3.110 g of TEA in a 100 mL three-necked bottle, add 20 mL of chloroform to dissolve them, and stir mechanically. Weigh 7.434 g of NPC in a round-bottomed flask, dissolve it with 35 mL of chloroform, and add it dropwise to the above-mentioned three-necked flask to react for 4 h. Precipitate with anhydrous ether to remove triethylamine hydrochloride, recover the supernatant and concentrate, wash three times with pH 3~4 phosphate buffer solution, and dry calcium chloride powder. The solvent was removed to obtain the product NPCEMA as a white solid, 7.287 g. CDCl 3 in solvent 1 H-NMR confirmed the structure of the product with a purity of 96%.
Embodiment 2
[0046] Embodiment 2: Contain the preparation of active ester functional polymer PMENG
[0047] The reaction process is as figure 1shown. Add 10 mL of absolute ethanol into a 100 mL three-necked flask, pass through nitrogen, stir with a magnetic force and gradually raise the temperature to 70 °C. Weigh 0.140 g of NPCEMA, 2.726 g of PEGA and 0.805 g of MPC in turn, and add 34 mL of absolute ethanol to dissolve them. Weigh 0.037 g AIBN and add it to the monomer mixture. Use the dropping funnel to drop the mixed solution of the monomers, and the dropwise addition is completed in about 2 hours. Change to a closed system and allow it to continue to react for 24 h. After the reaction was stopped, part of the reaction solution was taken out and put into a dialysis bag with a molecular weight cut off of 7000, and dialyzed in an acidic aqueous solution with a pH of 3~4. Freeze dried. D. 2 O solvent 1 H-NMR characterizes its structure, and it is composed of polymer PMENG with 12%...
Embodiment 3
[0048] Example 3: Grafting dopamine PMEDG, introducing a catechol group with adhesion function through the reaction of active ester and amino group
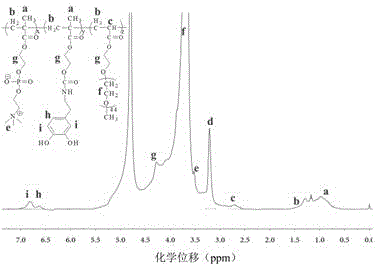
[0049] Continue to pass nitrogen in the three-necked flask of the previous step reaction, and gradually raise the temperature to 60°C in the oil bath. Weigh 0.145 g of dopamine into the above-mentioned three-neck flask, add 20 mL of ethanol, and adjust the pH of the reaction solution to about 7 with triethylamine, and react for about 12 h. Adjust the reaction solution to acidity, stop the nitrogen flow, transfer it to a dialysis bag with a molecular weight cut off of 7000, and dialyze it with an acidic aqueous solution with a pH of 3~4 for 3 days. Freeze dried. D. 2 O solvent use 1 H-NMR characterizes its structure. Such as figure 2 shown. The molar content of catechol groups, phosphorylcholine groups and polyethylene glycol chains were 8%, 64% and 28% copolymer PMEDG respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com