A serum-free chondrocyte culture medium
A technology of chondrocytes and culture medium, which is applied in the field of biomedical materials, can solve the problems of difficult clinical application of chondrocytes, accelerated dedifferentiation rate of chondrocytes, and limited amount of cartilage tissue, so as to maintain extracellular matrix secretion ability, good extracellular Matrix secretion ability, effect of promoting cell function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The obtained human cartilage tissue was obtained by enzymatic digestion to obtain primary chondrocytes, and the cell viability and quantity were calculated. After being washed with PBS, culture medium A (culture medium A was a mixed culture medium of DMEM and F12 at a volume ratio of 1:1, Then add 10% FBS) and the serum-free chondrocyte culture medium prepared in this example to resuspend, press 2×10 4 piece / cm 2 Inoculate into a culture bottle and place in an incubator at 37°C, 5% CO 2 Cultivate under the same conditions; change the medium after 48~72h, continue to cultivate, and change the medium every 48h thereafter. The adherence rate of primary human chondrocytes in serum-free group and serum group can reach about 60% within 48 hours, and subculture can be carried out when the confluence rate of chondrocytes reaches about 50%.
[0026] The serum-free culture medium used in this example is: the basic culture medium is a mixed culture medium of DMEM and F12 accordi...
Embodiment 2
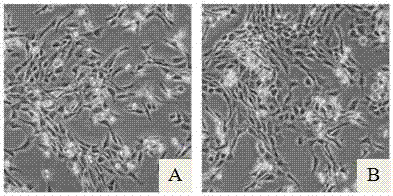
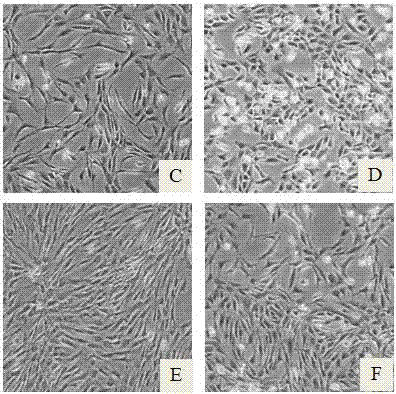
[0029] The obtained human cartilage tissue was digested overnight by enzyme digestion, the primary chondrocytes were collected by centrifugation, and suspended in culture medium A (culture medium A was a mixed culture medium of DMEM and F12 at a volume ratio of 1:1, and then added 10% FBS) After that, according to 0.5x10 4 / cm 2 Cell density seeded into cell culture flasks, placed at 37°C, 5% CO 2 Conditioned incubator for culture; when the cells reached 60%~80% confluence, the trypsin digestion method was used for subculture. The first group was cultured with culture medium A, and the second group was cultured with the serum-free culture medium of this example. Observation was carried out when passing to the 3rd and 6th generations.
[0030] The serum-free culture medium used in this example is: the basic culture medium is a mixed culture medium of DMEM and F12 according to the volume ratio of 1:1; add 6g / L dextran and stir to dissolve, and then add 0.5mL / L lipid concentrat...
Embodiment 3
[0033] The obtained human cartilage tissue was digested overnight by enzymatic digestion, and the primary chondrocytes were collected by centrifugation. After being suspended in culture medium B (culture medium B was DMEM basic culture medium, and 10% FBS was added), the 0.5x10 4 / cm 2 Cell density seeded into cell culture flasks, placed at 37°C, 5% CO 2 Culture in a conditioned incubator; when the cells reach 60% to 80% confluence, trypsin digestion is used for passage, and the serum-free culture medium of this example is used for culture.
[0034] The serum-free culture medium used in this example is: the basic culture medium is DMEM as the basic culture medium; add 15g / L dextran and stir to dissolve, then add 0.1mL / L lipid concentrate and 55mg / L pyruvic acid respectively Sodium, 0.5 μM T3, 0.1 mg / L hydrocortisone, 5 μg / L dexamethasone, 2 μg / L sodium selenite, 25 μM β-mercaptoethanol, 100 mg / L V C , 50 μg / mL glutamine, 25 μg / L bFGF, 10 μg / L EGF, 10 μg / L TGF-β, 5 μg / L PDGF,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com