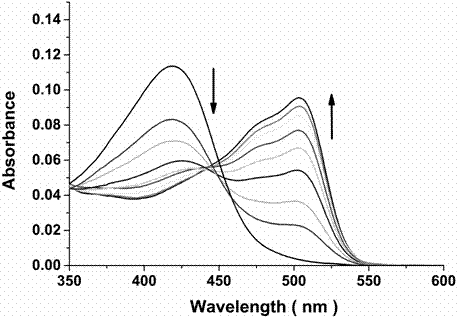
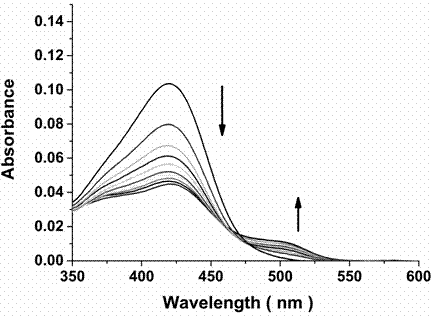
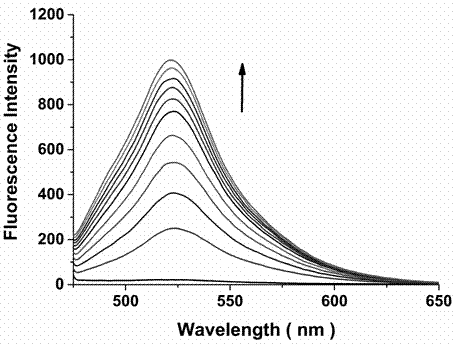
Bifunctional probe, its preparation method, and its application in detection of orthographic parallel conformation G-quadruplex
A dual-function, quadruplex technology, applied in the field of biological probes, can solve the problems of high price and cannot be widely used, and achieve the effects of simple preparation, stable structure and enhanced fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of compound 2
[0044] Dissolve 2.0g of 4-diethylamino salicylaldehyde in 30mL of absolute ethanol, add 3.20g of diethyl malonate and 1mL of piperidine, and react at 80°C for 6h. Then evaporate the solvent, add 20mL acetic acid and 20mL concentrated hydrochloric acid, continue to reflux for 6h, cool to room temperature, pour the reaction solution into ice water, adjust the pH to 5 with sodium hydroxide solution, precipitate a large amount of precipitate, vacuum filter and dry to obtain Crude. Purified by silica gel chromatography using petroleum ether / ethyl acetate (volume ratio 1 / 10) as the eluent to obtain 0.81 g of pure product 2 with a yield of 37.3%: 1 H NMR (400MHz, CDCl 3 )δ7.53(d, J=9.3Hz, 1H), 7.24(d, J=8.8Hz, 1H), 6.56(dd, J=8.8, 2.1Hz, 1H), 6.49(d, J=1.6Hz, 1H), 6.03(d, J=9.3Hz, 1H), 3.41(q, J=7.1Hz, 4H), 1.21(t, J=7.1Hz, 6H).ESI-MS m / z: 218.1[M+ H] + .
Embodiment 2
[0045] Embodiment 2: the synthesis of compound 3
[0046] Under nitrogen protection, 1.5mL POCl 3 Add dropwise to 1mL DMF and stir at room temperature for 20min. Then 0.77g of 2 dissolved in 4mL DMF was added dropwise to the above mixture, and reacted at 60°C for 10h. After cooling to room temperature, the reaction solution was poured into ice water, and the pH was adjusted to neutral with sodium hydroxide solution, and pumped under reduced pressure. Filter, wash with water and ethanol several times, dry in vacuo to obtain orange-yellow solid 30.50g, productive rate 58.3%: 1 H NMR (400MHz, CDCl 3 )δ10.13(s,1H),8.26(s,1H),7.41(d,J=9.0Hz,1H),6.64(dd,J=9.0,2.5Hz,1H),6.49(d,J=2.4 Hz,1H),3.48(q,J=7.1Hz,4H),1.26(t,J=7.1Hz,6H).ESI-MS m / z:246.1[M+H] + .
Embodiment 3
[0047] Embodiment 3: the synthesis of compound 5a
[0048] Dissolve 4,4'-difluorobenzil in DMSO (about 0.15M), add 3 times the molar amount of N-methylpiperazine and K 2 CO 3 , after heating and reacting in an oil bath at 90°C for 20h, add an appropriate amount of ether, wash away DMSO with water, and wash with anhydrous Na 2 SO 4 After drying, the solvent was evaporated and dried in vacuo to obtain a yellow solid with a yield of 95%; 1 HNMR (400MHz, CDCl 3 )δ7.85(d, J=9.0Hz, 4H), 6.85(d, J=9.1Hz, 4H), 3.41(t, J=5.0Hz, 2H), 2.53(t, J=5.0Hz, 2H) ,2.34(s,2H). 13 C NMR (101MHz, CDCl 3 )δ193.59, 154.84, 132.14, 123.32, 113.21, 54.58, 46.84, 46.05. MS (ESI+) m / z 407 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com