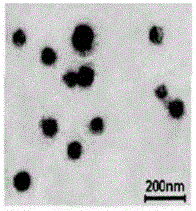
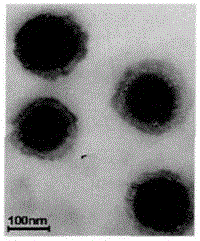
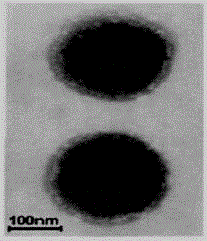
Preparation method of dihydromyricetin-carried ternary complex liposome
A technology of dihydromyricetin and ternary compounding, which is applied in liposome delivery, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of macrocytic toxicity and maintain biological activity , Improve transfection efficiency, reduce toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of dihydromyricetin-loaded ternary composite liposomes, comprising the following steps:
[0023] (1) Dissolve dihydromyricetin, phosphatidylglycerol (PG) and dioleoylphosphatidylethanolamine (DOPE) in chloroform at a molar ratio of 2:3:3, evaporate under reduced pressure to remove chloroform, and obtain dry lipid Membrane, add HEPES buffer (pH7.4), shake slowly to suspend the lipid film in HEPES buffer to obtain anionic liposome suspension, put the anionic liposome suspension in an ultrasonic cleaner for Ultrasound in a water bath, fully hydrated, and squeezed through the membrane to obtain anionic liposomes;
[0024] (2) Dissolve trimethyl-2,3-dioleoyloxypropylammonium bromide (DOTAP) and phosphatidylcholine (PC) in chloroform at a molar ratio of 1:1, and evaporate under reduced pressure to remove chloroform , to obtain a uniform film, add HEPES buffer (pH7.4), slow shaking makes the lipid film suspended in HEPES buffer, to obtain cationic liposom...
Embodiment 2
[0027] A preparation method of dihydromyricetin-loaded ternary composite liposome, said method comprising the following steps:
[0028] (1) Dissolve dihydromyricetin, phosphatidylinositol (PI) and cholesterol (CHOL) in chloroform at a molar ratio of 2.5:3.5:3.5, evaporate under reduced pressure to remove chloroform, obtain a dry lipid film, and add HEPES Buffer solution (pH7.4), shake slowly to suspend the lipid film in the HEPES buffer solution to obtain anionic liposome suspension, place the anionic liposome suspension in an ultrasonic cleaner for ultrasonic cleaning in a water bath, fully After hydration, squeeze through the membrane to obtain anionic liposomes;
[0029] (2) Dissolve 1,2-dioleoyl-3-succinyl-sn-glycerol choline ester (DOSC) and dioleoylphosphatidylethanolamine (DOPE) in chloroform at a molar ratio of 1:0.8, and decompress Evaporate and remove chloroform to obtain a uniform film, add HEPES buffer (pH7.4), slowly shake to suspend the lipid film in HEPES buffe...
Embodiment 3
[0032] A preparation method of dihydromyricetin-loaded ternary composite liposome, said method comprising the following steps:
[0033](1) Dissolve dihydromyricetin, phosphatidylserine (PS) and dioleoylphosphatidylethanolamine (DOPE) in chloroform at a molar ratio of 2.8:4:3, evaporate under reduced pressure to remove chloroform, and obtain dry lipid Membrane, add a certain volume of HEPES buffer (pH7.4), shake slowly to suspend the lipid film in HEPES buffer to obtain anionic liposome suspension, put the anionic liposome suspension in ultrasonic cleaning Ultrasound in a water bath in the instrument, after fully hydrating, squeeze through the membrane to obtain anionic liposomes;
[0034] (2) Dissolve trimethyl-2,3-dioleyloxypropyl ammonium chloride (DOTMA) and phosphatidylcholine (PC) in chloroform at a molar ratio of 1.2:1, and evaporate under reduced pressure to remove chloroform , to obtain a uniform film, add HEPES buffer (pH7.4), slow shaking makes the lipid film suspen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com