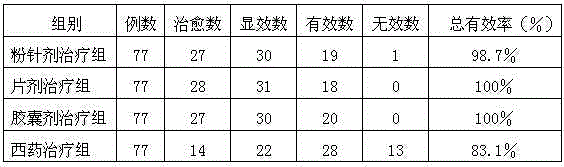
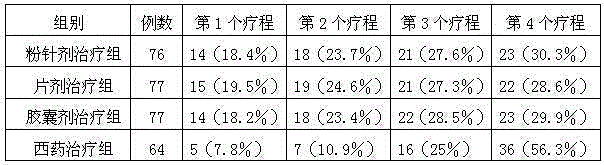
Medicinal preparation for treating deficiency cold of spleen and stomach type peptic ulcer and preparation method thereof
A technology for peptic ulcer and deficiency of the spleen and stomach, applied in the field of pharmaceutical preparations, can solve the problems of antibiotics with many toxic and side effects, unsatisfactory clinical efficacy, single treatment plan, etc., to improve immunity, protect gastric mucosa, and reduce gastric acid. secretory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Wherein, when the dosage form of described medicine is powder injection, its preparation method comprises the following steps:
[0077] In the first step, the raw medicinal materials are mixed in proportion, put into an ultrafine pulverizer and pulverized into a mixture composed of micron-sized particles with a volume average particle size of 0.1-10 μm and nano-sized particles with a volume-average particle size of less than 0.1 μm Powder, the powder yield of the mixed powder is at least 95%;
[0078] In the second step, add ethanol with an alcohol concentration of 85% to 95% relative to the mass of the mixed powder that is 3 to 5 times the mass of the mixed powder obtained in the first step, stir and dissolve to obtain an ethanol solution, and put the ethanol solution in Stand still for 24-36 hours under the condition of 5°C-10°C, use the percolation method to slowly percolate at a rate of 1-2ml per minute, collect the percolation liquid, concentrate and dry, and put i...
Embodiment 1
[0088] Embodiment 1 powder injection
[0089] The preparation process of the powder injection of the present invention is: take 250g of kohlrabi, 220g of halberd leaf stone reed, 260g of dried ginger, 200g of scopolamine, 190g of cinnamon, 240g of fennel, 150g of cumin, 180g of turnip, 170g of cloves, 160g of pepper, and powdered iron hoops. 190g, longan 220g, tangerine peel 240g, notopterygium 250g, woody fragrance 270g, black berry 240g, sandalwood 220g, lychee core 190g, Potentilla 230g, green calyx plum 210g, scallion 290g, panchang grass 250g, tianxian vine 320g , Qingxiangteng 180g, Gansong 240g, Persimmon stalk 260g, Valerian 220g, Divine Comedy 140g, Hawthorn 210g, Rice bud 240g, Bamboo leaf lotus 260g, Raisin 230g, Ferulicum 190g, Betel nut 200g, Pachymodium 220g, Astragalus 300g, 180g of Atractylodes macrocephala, 200g of crape myrtle leaves, 230g of yam, 20g of zucchini grass, 250g of white lentils, 270g of rhodiola rosea and 260g of seabuckthorn;
[0090] In the f...
Embodiment 2
[0093] Example 2 tablet
[0094] The preparation process of oral tablet of the present invention is: get 280g of kohlrabi, 180g of halberd leaf stone reed, 240g of dried ginger, 320g of scopolamine, 230g of cinnamon, 280g of fennel, 210g of cumin, 200g of turnip, 140g of clove, 170g of pepper, iron hoop 120g powder, 240g longan, 220g tangerine peel, 260g notopterygium, 320g woody fragrance, 300g black berry, 220g sandalwood, 240g lychee core, 250g green pottery, 220g green calyx plum, 280g scallion, 230g pangus, celestial vine 330g, Qingxiangteng 190g, Gansong 260g, Persimmon stalk 290g, Valerian 220g, Divine Comedy 200g, Hawthorn 260g, Rice bud 250g, Bamboo leaf lotus 290g, Radix radish 240g, Asafoetida 280g, Betel nut 160g, Pachymos 320g, Astragalus 330g, Atractylodes macrocephala 200g, crape myrtle leaves 210g, yam 230g, Zishikoucao 10g, white lentils 140g, rhodiola rosea 330g and seabuckthorn 300g;
[0095] The first step is to mix the various raw materials of the drug in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com