Novel triterpene compound in potentilla anserina, and preparation method and application thereof
A technology of triterpene compounds and compounds, applied in the direction of steroids, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems that have not yet been reported on triterpene compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
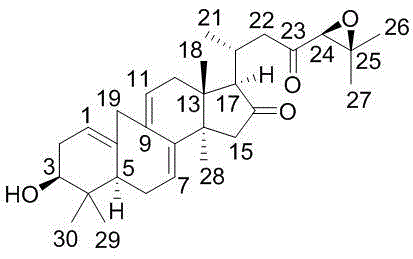
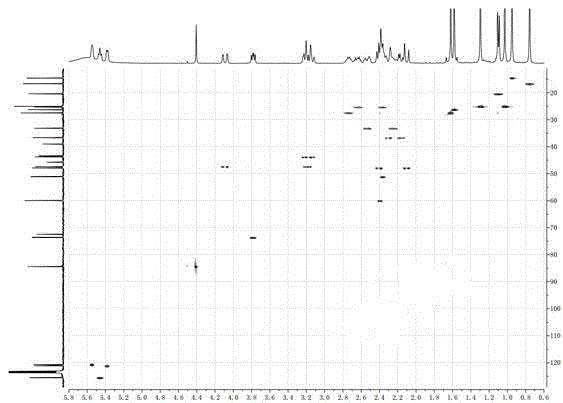
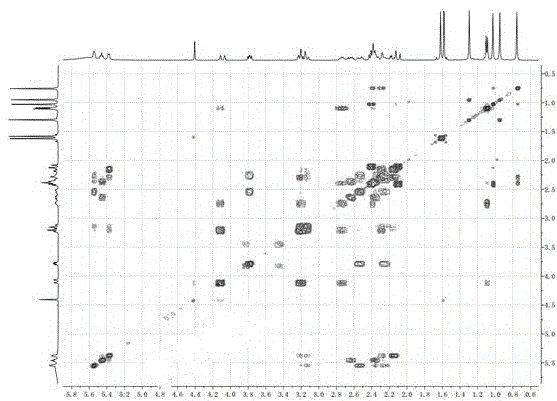
[0029] Example 1: Isolation, Preparation and Structure Confirmation of Compound I
[0030] (a) Crack the rhizome (10kg) and extract it with 75% ethanol under heat reflux (30L×3 times), combine the extracts, concentrate until there is no alcohol smell (6L), and then use petroleum ether (6L×3 times), acetic acid Ethyl ester (6L×3 times) and water-saturated n-butanol (6L×3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract (375g) and n-butanol extract respectively; (b) step (a ) in the ethyl acetate extract was removed with D101 macroporous resin, eluted with 15% ethanol (8L) and 70% ethanol (12L) in turn, collected 70% eluate, concentrated under reduced pressure to obtain 70% ethanol eluted concentration (125g); (c) The concentrate eluted with 70% ethanol in step (b) was separated with normal-phase silica gel, and dichloromethane with a volume ratio of 40:1, 20:1, 10:1 and 5:1 was used successively - Gradient elution with methanol yielded 4 component...
Embodiment 2
[0032] Embodiment 2: Compound I pharmacological action test
[0033] 1. Test method
[0034] 1. Cell culture
[0036] The A375 melanoma cells frozen in the liquid nitrogen tank were taken out, quickly placed in warm water at 37°C, and gently shaken to melt as soon as possible (about 1 min). Then suck out the cell suspension, add it to the centrifuge tube that has been added with 2mL of medium, gently blow and mix, centrifuge at 800r / min for 5min, and discard the upper layer of medium. The cell suspension was prepared with 8 mL of DMEM medium containing 10% FBS and 1% Penicillium G / Streptomycin, and then inoculated into a 10 cm culture dish. Then placed in 5% CO 2 , and cultured in a 37°C constant temperature incubator.
[0037] 1.2 Cell passage
[0038] Observe under a microscope that the cell confluency reaches 80%-90% and can be passaged. Wash the cells twice with 2 mL of PBS, and then add 1 mL of 0.25% trypsin. Gently shake the culture pla...
Embodiment 3
[0084] Preparation of tablets: Compound I was first prepared according to the method in Example 1, and salts made from organic acids such as tartaric acid, or citric acid, formic acid, or oxalic acid, and inorganic acids such as hydrochloric acid, sulfuric acid, or phosphoric acid. The weight ratio of excipients is 1:7, adding excipients, granulating and compressing into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com