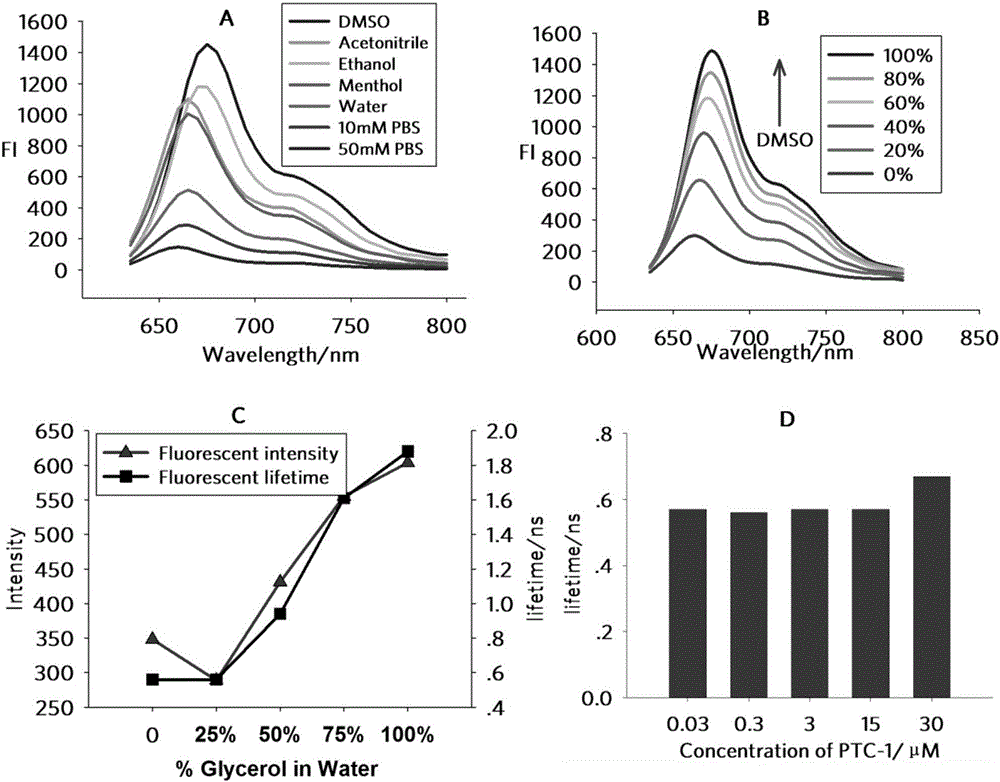
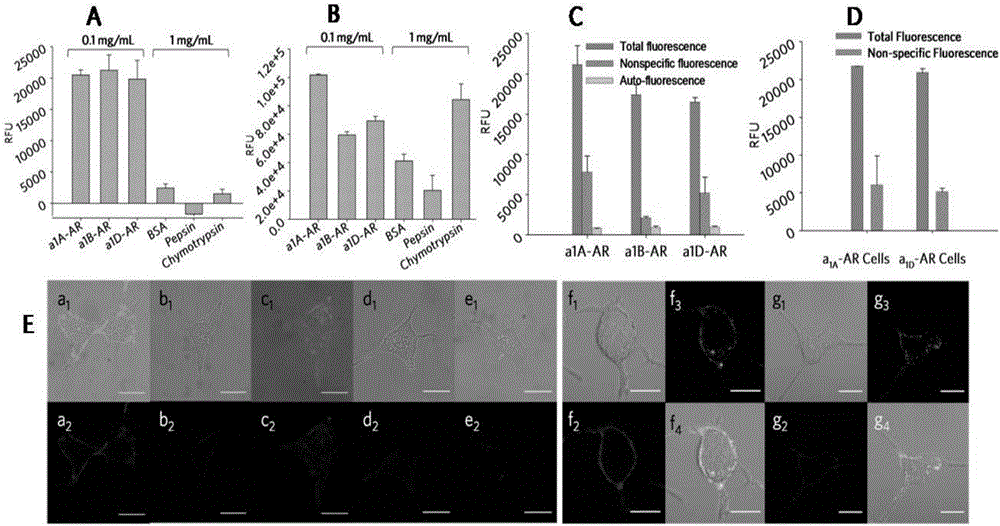
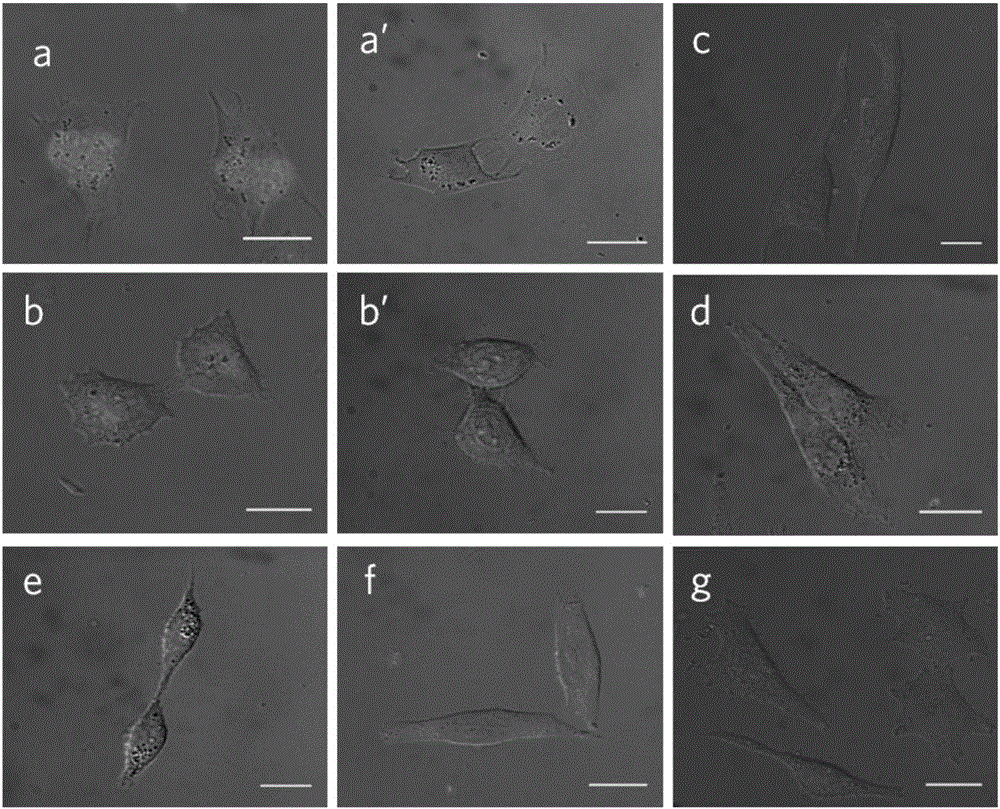
Environment-sensitive alpha1-adrenergic receptor near infrared fluorescence ligands and application thereof
An adrenaline and environment-sensitive technology, applied in the direction of fluorescence/phosphorescence, preparations for in vivo tests, luminescent materials, etc., can solve the problems of few applications and no selectivity of this coloring, so as to improve the signal-to-noise ratio and provide Sensitivity, effects of avoiding washing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Example 1: Synthesis of PTC-1~3
[0031]
[0032] Reagents and conditions: (a) 3-iodopropionic acid, acetonitrile, reflux, 48h, 81%; (b) methyl iodide, acetonitrile, reflux, 12h, 86%; (c) hydrochloric acid-N-(3-benzene Amino-2-propenylidene)aniline, AcOH / AC 2 O, 120℃, 4h; Pyridine / AcOH, 120℃, 4h; 30% two steps; (d) propargylamine, EDCI, DMAP, dichloromethane, r.t., 6h, 53%; (e) piperazine, H 2 O, 100°C, 81%; (f) acid chloride, TEA, acetonitrile, 1h, 0°C, 62-75%; (g) NaN 3 , DMSO, 100℃, 6h, 35-88%; (h) CuSO4, sodium ascorbate, tBuOH / H 2 O=2:1, 50°C, 1h, 48.9-67.3%.
[0033] Intermediate C3: 1-(2-carboxyethyl)-2,3,3-trimethyl-3H-indole-1-iodonium
[0034] 2,3,3-Trimethyl-3H-indole (1.0g, 6.23mmol) and 3-iodopropionic acid (1.86g, 9.3mmol) were dissolved in 15mL of acetonitrile, refluxed for 48 hours, and cooled to room temperature. After the solvent was spin-dried, 100 mL of ethyl acetate was slowly added under ultrasonic vibration, and a large amount of precipita...
example 2
[0061] Example 2: Synthesis of PTC-4~6
[0062]
[0063] Reagents and conditions: (a) H 2 O / acetonitrile,NaN 3 , 80℃, 20h, 43-74%; (b) TsCl, triethylamine, chloroform, r.t., 24h, 65-88%; (c) K 2 CO 3 , acetonitrile, 90°C, 64-69%; (d) CuSO4, sodium ascorbate, tBuOH / H 2 O=2:1, 50°C, 1h, 55.2-72.5%.
[0064] Intermediate 11a: 2-Azidoethanol
[0065] NaN 3 (3.12g, 48mmol) was carefully added to a mixed solution of 2-bromoethanol (2g, 16mmol) in acetonitrile (10mL) and water (40mL), stirred vigorously at 80°C for 20h, then cooled to room temperature. The reaction solution was extracted with dichloromethane (3×50 mL), dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off to obtain 0.7 g of a transparent oil with a yield of 50%. 1 HNMR (300MHz, CDCl3): δ3.80 (td, 2H, J 1 =4.8Hz,J 2 = 3.0Hz), 3.27(t, 2H, J = 5.4Hz), 1.96(s, 1H).
[0066] Intermediate 11b: 3-Azido-1-propanol
[0067] Synthesis of intermediate 11b Using 3-bromo-1-propanol as r...
example 3
[0088] Example three: the synthesis of PHC-1~3
[0089]
[0090] Reagents and conditions: (a) K 2 CO 3 , acetonitrile, 90°C, 73-96%; (b) CuSO4, sodium ascorbate, tBuOH / H 2 O=2:1, 50°C, 1h, 49.4-57.7%.
[0091] Intermediate PH1: 1-(2-azidoethyl)-4-(2-methoxyphenyl)piperazine
[0092] To the acetonitrile solution (30 mL) of 1-(2-methoxyphenyl)piperazine (665 mg, 3.46 mmol) and 11a (918 mg, 3.8 mmol) was added 1.43 g of potassium carbonate, and the mixture was refluxed for 24 h, then filtered immediately to obtain the filtrate. The solvent was evaporated and purified by silica gel column chromatography to obtain 690 mg of yellow oil with a yield of 76%. 1 HNMR (300MHz, CDCl 3 ): δ7.02-6.84(m,4H), 3.80(s,3H), 3.41(t,2H, J=6.0Hz), 3.11(m,4H), 2.73-2.65(m,6H); ESI- MS:m / z[M+H] + calcdforC 13 h 20 N 5 o + 262.2, found 262.4.
[0093] Intermediate PH2: 1-(2-azidopropyl)-4-(2-methoxyphenyl)piperazine
[0094] Synthesis of intermediate P7 Using intermediate 10b (906mg, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com