Acetophenone type photoinitiators using fluorene as conjugated structure as well as preparation method and application of acetophenone type photoinitiators
A technology of photoinitiator and conjugated structure, which is applied in the field of preparation of substituted acetophenone-based photoinitiators, can solve the problems of small molar extinction coefficient, weak absorption, and restrictions on the wide application of photoinitiators, and achieve easy dissolution, fast High initiation rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
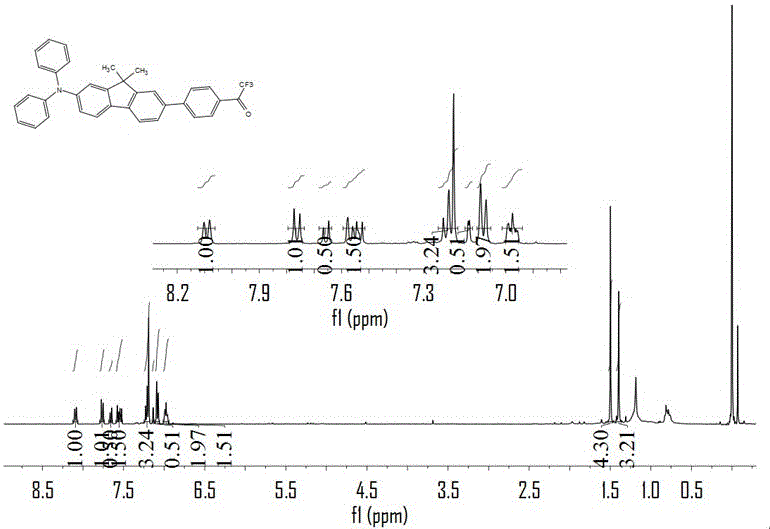
[0044] (1) Synthesis of 4,4-(N,N diphenylamino) 4-bromo-9,9-dimethylfluorene
[0045] Diphenylamine (33.6g, 0.2mol), 2,7-dibromo-9,9-dimethylfluorene (91.0g, 0.255mol), copper powder (6.4g, 0.1mol), potassium carbonate (56g, 0.4mol), potassium iodide (6.0g, 0.036mol), 18-crown-6 (6.0g, 0.025mol), ortho-dichlorobenzene (200g, 1.36mol) were added to a 500mL flask, vacuum-gas three times System placed at 200 o Heat and reflux in a C oil bath for 96 hours in the dark. After the reaction, o-dichlorobenzene was distilled off under reduced pressure, washed with petroleum ether, and by-products were removed by suction filtration. After the petroleum ether was removed by rotary evaporation, it was extracted three times with dichloromethane, and the organic phase was dried with anhydrous sodium sulfate. v:v), a white powder was obtained after rotary evaporation. Drying in a vacuum oven for 24 hours, the product was 32.0g of 4,4-(N,N diphenylamino)4-bromo-9,9-dimethylfluorene, The yi...
Embodiment 2
[0051] The preparation method is the same as in Example 1, except that in the process of preparing the photoinitiator, 4-bromoacetophenone is used to replace 4-bromo-2,2,2 trifluoroacetophenone to prepare a photoinitiator molecule containing a methyl structure , the molecular structure is the target molecule 2.
Embodiment 3
[0052] Embodiment 3 photoinitiated polymerization process is as follows:
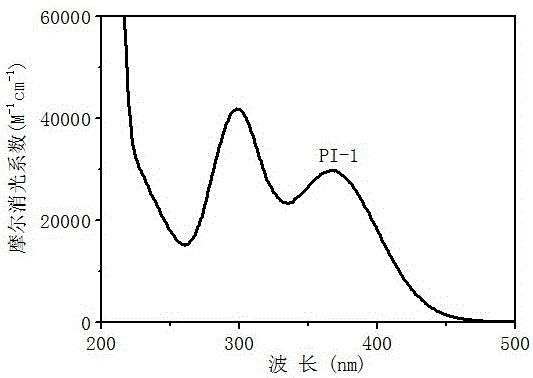
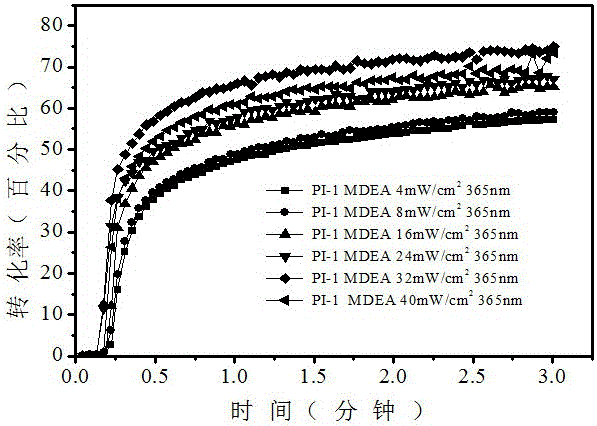
[0053] (1) Free radical polymerization, first prepare the photoinitiation system, monomer (trimethylolpropanetriacrylate, TMPTA): photoinitiator 1: auxiliary agent (N-vinylcarbazole, NVK) = 100: 1.5: 4.5 / 0 (m: m: m) Stir again to fully dissolve. At 4~48mW / cm 2 Under the irradiation of 365nm light sources with different light intensities, the polymerization kinetics and the final monomer conversion rate were speculated by the change of the characteristic peak of the monomer by the method of online infrared spectrum monitoring.
[0054] (2) Cationic polymerization, first prepare photoinitiation system, monomer epoxy cyclohexane: photoinitiator: iodonium salt = 100: 1.5: 4.5 / 0 (m: m: m) and then stir to fully dissolve. Under the irradiation of 365nm light source with different light intensity from 4 to 48mw, the conversion rate of polymerization was estimated by the change of characteristic peak of monom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com