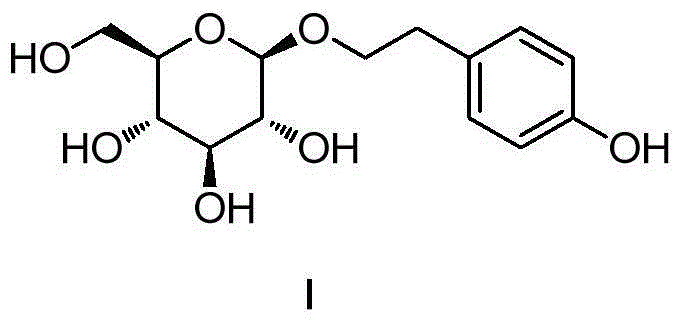
Synthesis method for salidroside and intermediate compound obtained in synthesis method
A synthesis method and technology of salidroside, which are applied in the directions of esterification saccharides, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult glycosidation reaction, high time and cost consumption, and no protection of phenolic hydroxyl groups. , to achieve the effect of simplifying the operation steps and processing technology, the safety of human medicine, and avoiding by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
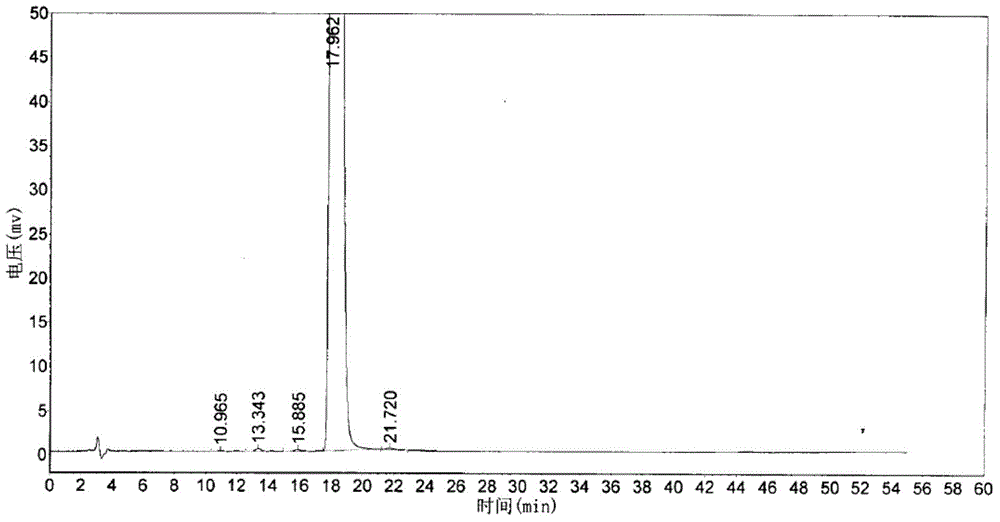
Image
Examples
Embodiment 1
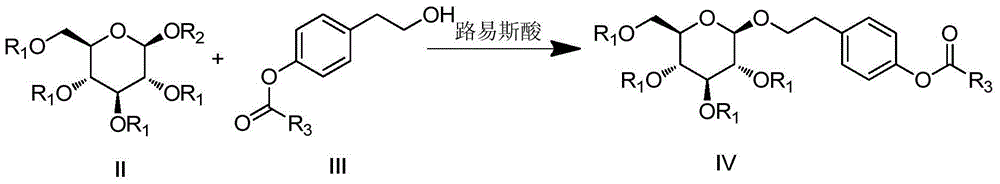
[0061] (1) Intermediate II (R 1 is pivaloyl, R 2 for the synthesis of trifluoroacetyl)
[0062] Add 60g (0.1mol) of penta-pivaloyl glucose, 300ml of acetic acid, 21g of trifluoroacetic anhydride, and 40ml of boron trifluoride ether into a 500ml three-necked flask, heat and reflux for 10h, after the reaction is completed, cool down, pour into 1L of water for crystallization, and filter , washed with water until neutral, and dried under reduced pressure at 60°C to obtain 53.3g of a white solid with a yield of 87.1%. 1 H-NMR (300MHz, CDCl 3 ): δ=6.42~6.40(d,1H), 5.24~5.79(m,2H,CH), 3.73~3.62(m,1H,CH), 4.27~4.14(m,1H,CH), 3.46~2.94( m,2H), 2.56~2.48(m,4H,CH), 1.12(s,36H,CH 3 ).
[0063] (2) Synthesis of compounds shown in formula IV (the phenolic hydroxyl group of formula III is protected by benzoyl group, i.e. compound 9)
[0064] Add 33.4g (0.06mol) of intermediate II, 10.4g (0.05mol) of intermediate III, and 120ml of acetonitrile into a 500ml three-necked flask, and add 1...
Embodiment 2
[0070] (1) Intermediate II (R 1 is pivaloyl, R 2 for the synthesis of trifluoromethanesulfonyl)
[0071] Add 60g (0.1mol) of pivaloyl glucose, 300ml of acetic acid, 28.2g of trifluoromethanesulfonic anhydride, and 40ml of boron trifluoride ether into a 500ml three-necked flask, heat and reflux for 10h, after the reaction is completed, cool, pour into 1L of water for crystallization, Filter, wash with water until neutral, and dry under reduced pressure at 60°C to obtain 59.4 g of white solid, yield 94%, 1 H-NMR (300MHz, CDCl 3 ): δ=6.82(d,1H), 5.14~5.57(m,2H,CH), 3.23~3.16(m,1H,CH), 4.17~4.08(m,1H,CH), 3.41~2.84(m, 2H), 1.22(s,36H, CH 3 ).
[0072] (2) Synthesis of compounds shown in formula IV (the phenolic hydroxyl group of formula III is protected by isobutyryl, i.e. compound 8)
[0073] Add 38g (0.06mol) of intermediate II, 10.4g (0.05mol) of intermediate III, 120ml of dichloromethane, 2.8g of zinc chloride into a 500ml three-neck flask, react at 25-30°C for 8h, and p...
Embodiment 3
[0077] (1) Synthesis of intermediate II (R1 is isobutyryl, R2 is trifluoromethanesulfonyl)
[0078] Add 53g (0.1mol) of pentaisobutyrylglucose, 300ml of acetic acid, 28.2g of trifluoromethanesulfonic anhydride, and 35ml of boron trifluoride ether into a 500ml three-neck flask, heat and reflux for 10h, after the reaction is completed, cool, pour into 1L of water for crystallization , filtered, washed with water until neutral, and dried under reduced pressure at 60°C to obtain 53.5 g of a white solid with a yield of 93%. 1 H-NMR (300MHz, CDCl 3 ): δ=6.64~6.59(d, 1H, CH), 5.34~5.29(m, 2H, CH), 3.71~3.61(m, 1H, CH), 4.23~4.16(m, 1H, CH), 3.45~ 2.94(m,2H), 2.56~2.48(m,4H,CH), 1.32(d,24H,CH 3 ).
[0079] (2) Synthesis of compounds shown in formula IV (the phenolic hydroxyl group of formula III is protected by isobutyryl group, i.e. compound 2)
[0080] Add 34.6g (0.06mol) of intermediate II, 11.8g (0.05mol) of intermediate III, 120ml of nitromethane, and 3.5g of zinc bromide int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com