A kind of flunarizine hydrochloride matrix sustained-release tablet and preparation method thereof
A technology of flunarizine hydrochloride and sustained-release tablets, which can be applied to pharmaceutical formulas, medical preparations containing active ingredients, extracellular fluid diseases, etc., and can solve the problem of short maintenance time of effective blood drug concentration and unstable drug effects , easy to produce irritation and other problems, to achieve the effect of maintaining blood concentration, improving bioavailability, and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
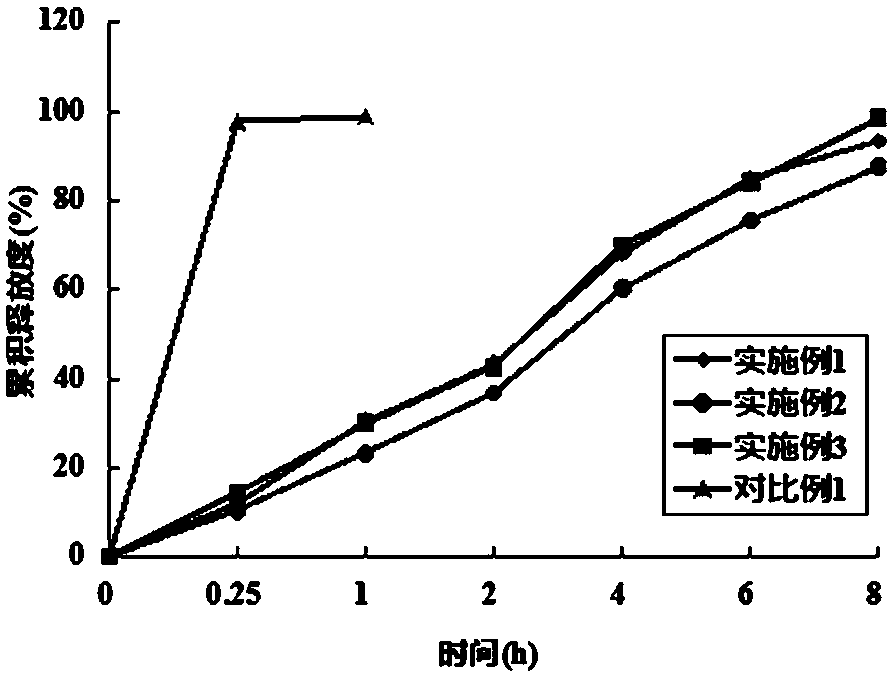
Embodiment 1
[0024] The flunarizine hydrochloride matrix sustained-release tablet in this embodiment is a hydrophilic gel matrix sustained-release tablet, and the sustained-release tablet is made of the following components in parts by weight: 35 parts of flunarizine hydrochloride, matrix material (hydroxyl Propyl methyl cellulose) 45 parts, diluent (lactose) 10 parts, binder (95% ethanol) 5 parts, lubricant 5 parts (magnesium stearate 2 parts, talc powder 3 parts).
[0025] Its preparation method is: pulverize flunarizine hydrochloride and pass through a 120-mesh sieve, mix evenly with hydroxypropyl methylcellulose and lactose, add ethanol solution with a volume fraction of 95% to moisten, and then granulate, 45-55 ℃ drying, passing through a 24-mesh sieve for granulation, then adding magnesium stearate and talc powder, mixing evenly, and directly pressing into tablets to obtain flunarizine hydrochloride matrix sustained-release tablets.
Embodiment 2
[0027] The flunarizine hydrochloride matrix sustained-release tablet in this embodiment is an inertable matrix sustained-release tablet, and the sustained-release tablet is made of the following components by weight: 35 parts of flunarizine hydrochloride, matrix material (ethyl Cellulose) 45 parts, diluent (lactose) 10 parts, binder (povidone) 5 parts, lubricant 5 parts (magnesium stearate 2 parts, talc powder 3 parts).
[0028] Its preparation method is: pulverize flunarizine hydrochloride and pass through a 120-mesh sieve, mix evenly with lactose, ethyl cellulose, povidone, magnesium stearate and talcum powder, and directly compress the whole powder into tablets to obtain fluorine hydrochloride Cinnarizine Matrix Extended Release Tablets.
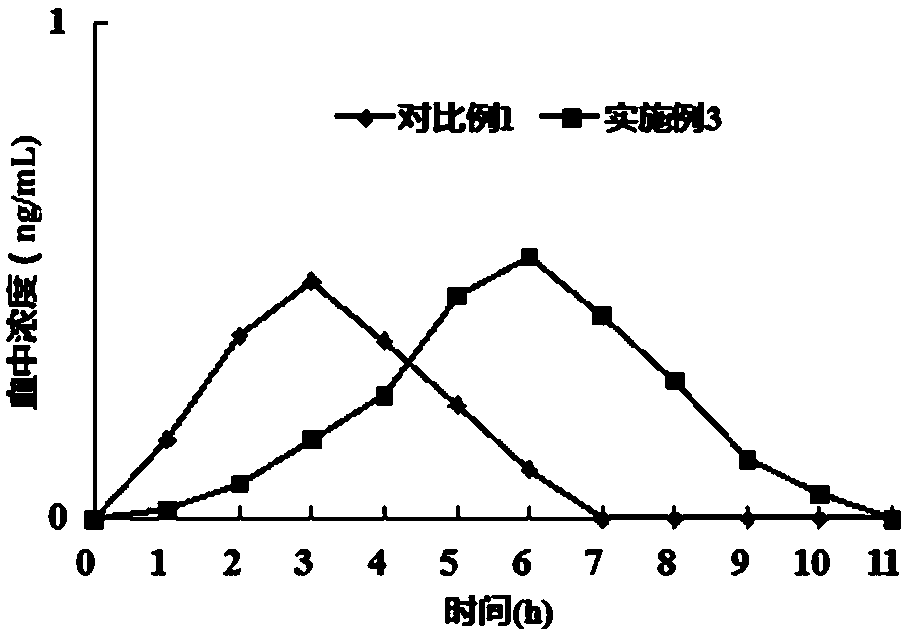
Embodiment 3
[0030] The flunarizine hydrochloride matrix sustained-release tablet in this example is a mixed matrix sustained-release tablet, which is made of the following components by weight: 40 parts of flunarizine hydrochloride, 45 parts of matrix material (hydroxyl Propyl methyl cellulose 35 parts, glyceryl monostearate 10 parts), diluent (lactose) 5 parts, binder (hypromellose) 5 parts, lubricant 5 parts (magnesium stearate 2 parts, talcum powder 3 parts).
[0031] The preparation method is as follows: pulverize flunarizine hydrochloride, pass through a 120-mesh sieve, mix with lactose, hydroxypropyl methylcellulose, glyceryl monostearate, hypromellose, magnesium stearate and talcum powder Evenly, the whole powder is directly compressed into tablets to obtain flunarizine hydrochloride matrix sustained-release tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com