Method for quantitative determination of catechol-o-methyl transferase activity and use thereof
A methyltransferase and quantitative determination technology is applied in the field of quantitative determination of catechol-O-methyltransferase activity, which can solve the problems of inconvenient quantitative detection, difficult separation by liquid chromatography, high cost and the like, and achieves good fluorescence emission. The effect of spectral characteristics, simple synthesis process and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 4-Acetate-7-Hydroxy-8-Methoxycoumarin
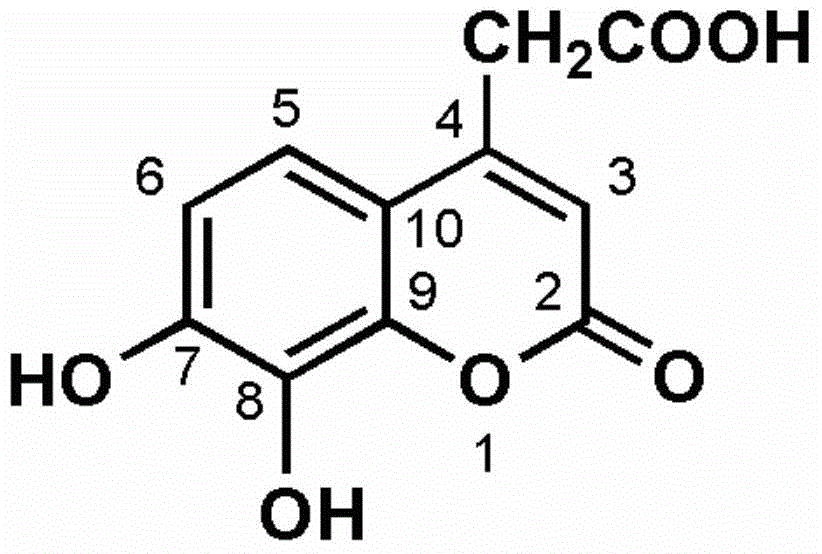
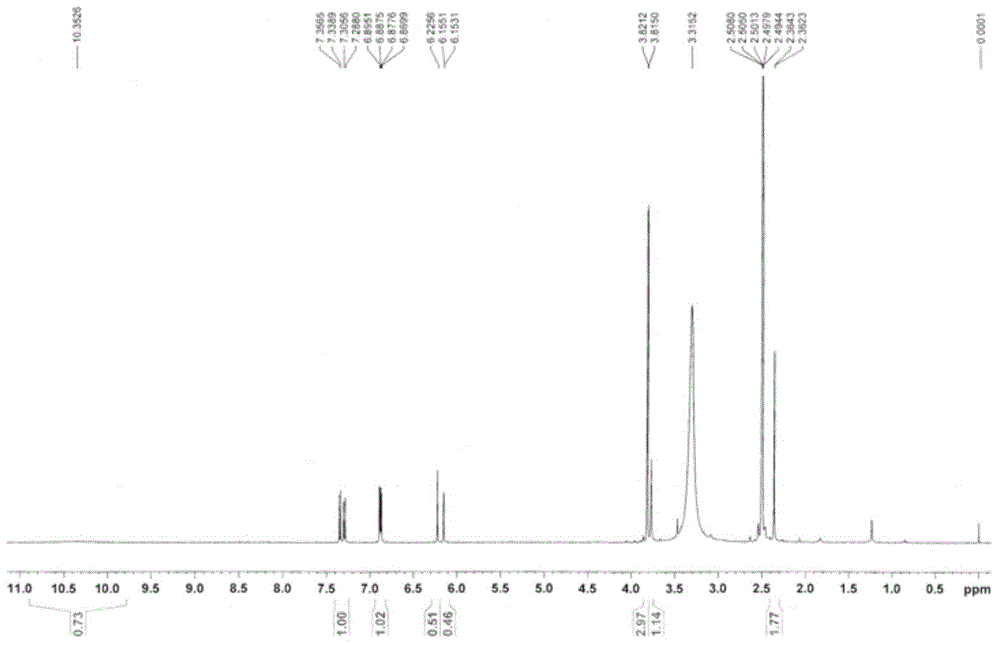
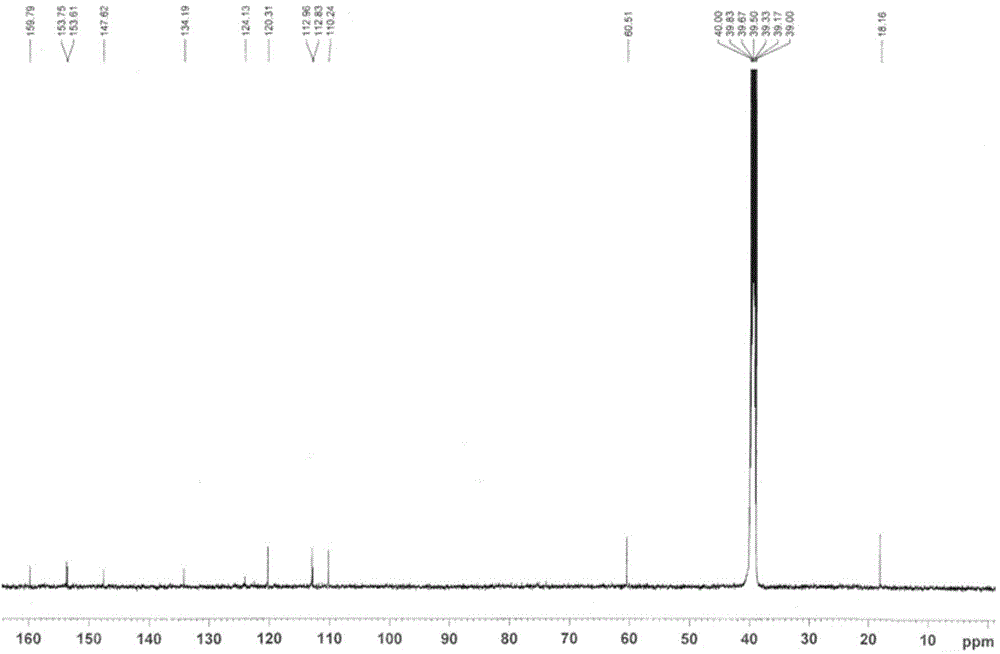
[0039] The synthetic route of 4-acetic acid-7-hydroxyl-8-methoxycoumarin is as follows Figure 8 As shown, weigh 0.5g of 2-methoxyresorcinol and place it in a three-necked flask, add 0.6g of acetone-1,3-malonic acid, 1.5ml of perchloric acid, stir at room temperature for 20 minutes, and heat to 55 °C, react for 3 hours, TLC detects that the reaction is complete, the reaction solution is directly filtered, and the filter cake is collected, which is the crude product. The crude product was subjected to silica gel column chromatography to obtain 150 mg of 4-acetic acid-7-hydroxy-8-methoxycoumarin as a white solid. Structural formula such as figure 1 show its 1 H-NMR spectrum and 13 C-NMR spectrum as figure 2 with image 3 shown;
[0040] 1 HNMR (500MHz, d-DMSO) δ: 3.31 (s, 2H, CH2), 3.82 (s, 3H, OCH3), 6.22 (s, 1H, COCH=C), 6.89 (d, J=3Hz, 1H, ArH ), 7.34 (d, J=8Hz, 1H, ArH);
[0041] 13 CNMR (500MHz, d-DMSO...
Embodiment 2
[0043] Quantitative assessment of COMT activity in liver microsomes from different individual sources
[0044] (1) Select 12 cases of human liver microsomes (HLM) and dilute them to 10mg / ml to prepare the COMT metabolic reaction system, including Tris-HCl buffer (50mM) at pH 7.4, human liver microsomes (0.5mg / ml), Dithiothreitol 40mM, MgCl 2 50mM, the final concentration of 4-acetic acid-7,8-dihydroxycoumarin is 5μM, pre-incubated with shaking for 3 minutes at 37°C;
[0045] (2) Add 10 μl of SAM with a concentration of 4 mM to the reaction system to initiate the reaction;
[0046] (3) After 15 minutes, add 200 μl of glacial acetonitrile, shake vigorously, and terminate the reaction;
[0047] (4) Use a high-speed refrigerated centrifuge at 4°C, 20,000×g, after high-speed centrifugation for 20 minutes, take the supernatant, perform fluorescence detection (Ex=320nm, Em=520nm), and substitute the obtained fluorescence intensity into the standard curve Afterwards, the metabolic ...
Embodiment 3
[0049] Determination of the lower limit of detection of COMT in vitro
[0050] The experiment was carried out on a microplate reader using a 96-well plate, 4-acetate-7,8-dihydroxycoumarin 5μM, S-adenosylmethionine 200μM, dithiothreitol 2mM, MgCl 2 5mM, COMT single enzyme 5ng / ml~100ng / ml, pH7.4 Tris-HCl buffer 50mM, total volume 100μL, incubated at 37°C for 1h and analyzed by microplate reader, the average value of each group was compared with that without COMT Compared with the control group, it was shown that 30 and 50ng of COMT were statistically significant (P<0.05), and the lower limit of detection of COMT was determined to be 30ng.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com