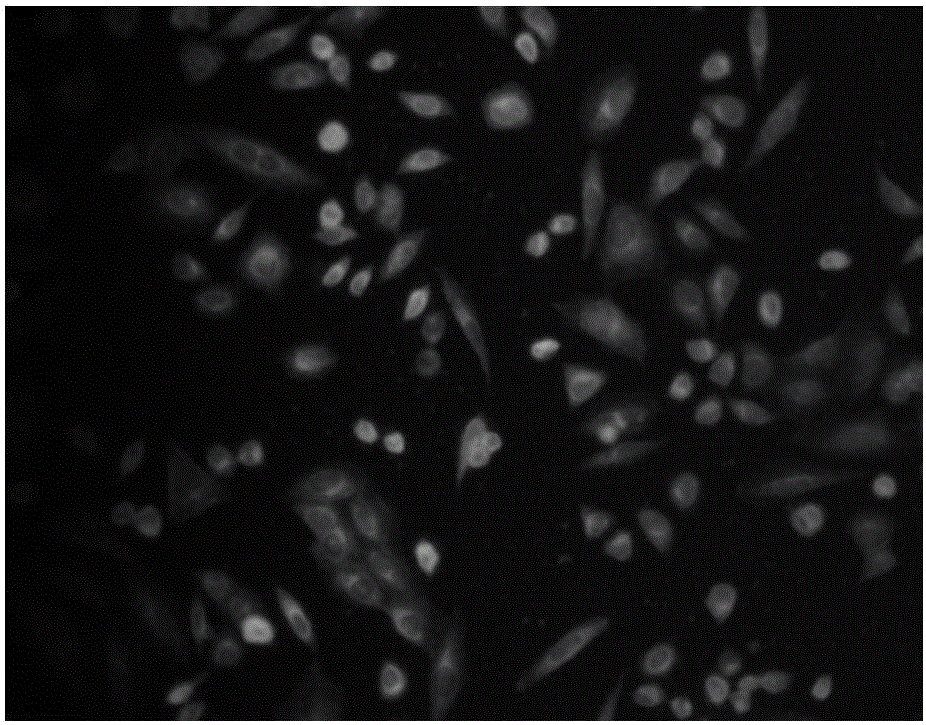
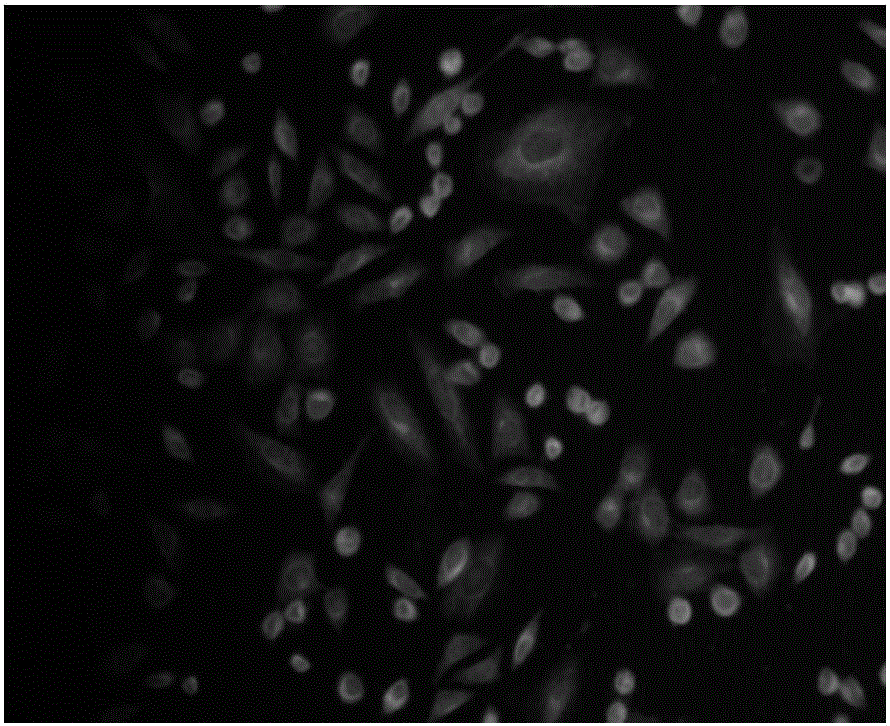
Preparation method and application of mercaptan fluorescence probe based on coumarin
A technology of coumarin hydrazide and fluorescent probe, which is applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems such as difficult to distinguish, and achieve low cytotoxicity, good selectivity, and good photochemical stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The present invention will be described in detail below in conjunction with the accompanying drawings.
[0036] The instruments and reagents used in the present invention were obtained from commercial sources unless otherwise specified.
[0037] 1 Experimental part
[0038] 1.1 Materials and reagents
[0039] p-tert-butylphenol, trifluoroacetic acid, 4-diethylamino salicylaldehyde, piperidine, absolute ethanol, diethyl malonate, dichloromethane, ethyl acetate, acetonitrile, hydrazine hydrate, hexamethylene Tetramine, NaOH, HCl, anhydrous Na 2 SO 4 , L-cysteine, N-acetyl-cysteine, L-homocysteine, glutathione, L-tryptophan, L-glycine, L-lysine, L-histidine Acid, L-alanine, L-valine, L-proline, L-phenylalanine, methionine, serine, threonine, aspartic acid, arginine, leucine, isoleucine All the above reagents were of analytical grade. Fetal bovine serum, penicillin, DMEM medium.
[0040] 1.2 Main instruments
[0041] Three-purpose UV Analyzer, UV-Vis Spectrophotomet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com