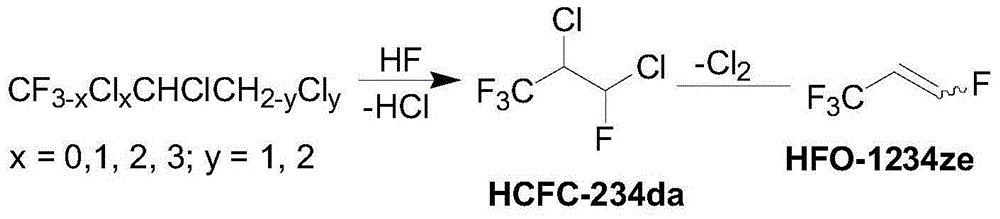
Preparation method of 1,3,3,3-tetrafluoropropene
A technology of tetrafluoropropene and tetrafluoropropane, which is applied in the field of preparation of hydrofluoroolefins, can solve the problems of harsh reaction conditions, expensive raw materials, and expensive prices, and achieve the effects of mild reaction conditions, cheap and easy-to-obtain raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]Add trichloropropene (20.0 g, 0.15 mol) and 120 mL of chloroform successively to a 250 mL dry there-necked flask equipped with magnetic stirring, a thermometer, and a condensing device, and add anhydrous aluminum trichloride ( 2.0g, 0.015mol), then gradually raised the temperature to 60°C, reacted for 12h, cooled the reaction solution to about 30°C, filtered, distilled the filtrate under reduced pressure, and collected the fraction at 120-125°C under a vacuum of 5kPa, which is 1,1,1,2,3,3-hexachloropropane (HCC-230da), conversion rate 85.7%, selectivity 90.4%.
Embodiment 2
[0030] Catalyst preparation: a certain proportion of Zn(NO 3 ) 2 ·6H 2 O, FeCl 3 , La(NO 3 ) 3 ·6H 2 Mix O into a 2mol / L aqueous solution, then add dropwise ammonia water with a mass fraction of 15% under constant stirring at 20°C-40°C, adjust the pH to about 8.0, react for 8 hours, filter, and then dry at 120°C for 2 hours , then mix a certain amount of magnesium oxide with it evenly, and then roast at 200°C for 1h, raise it to 320°C at 5°C / min, roast for 2h, then rise to 450°C at 10°C / min, roast for 4h, and finally pass hydrogen fluoride at 200°C The Zn-Fe-La-Mg composite catalyst was obtained by activation treatment at ℃-380℃ for about 36 hours.
[0031] Inside diameter is in the fixed-bed tubular reactor of 38mm, packs 50mlZn-Fe-La-Mg composite catalyst, wherein the mol ratio of Zn, Fe, La, Mg four is 0.5:2:0.5:7, and to catalyst Dry, and then at 250 ° C, HF and CCl 3 CHClCHCl 2 (HCC-230da, abbreviated as 230da) is passed into the first reactor R1, the molar ratio...
Embodiment 3~5
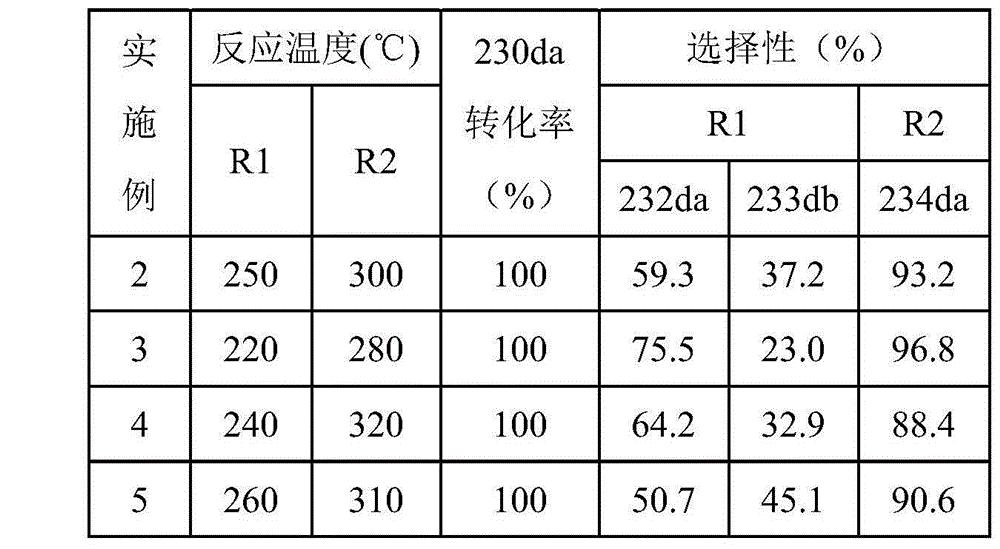
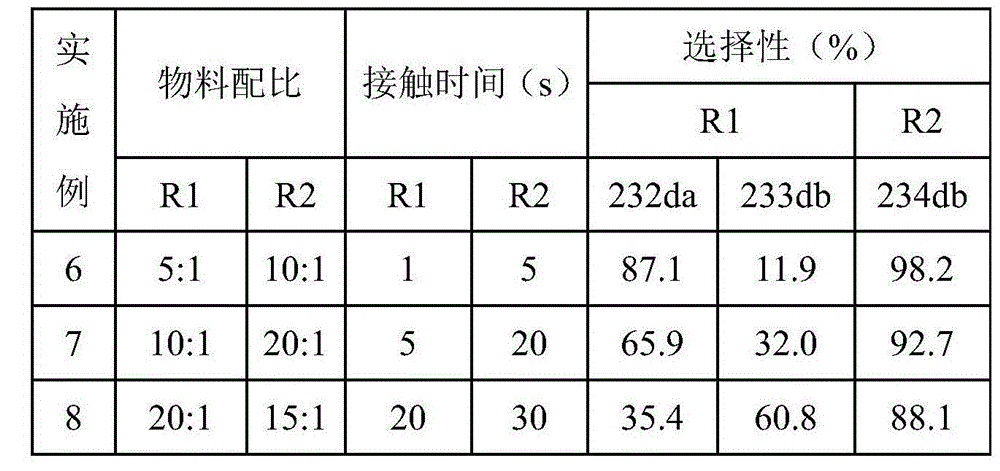
[0033] Embodiments 3-5 prepared HCFC-234da according to the same method as in Example 2, except that the reaction temperatures of R1 and R2 in Example 2 were 250°C and 300°C respectively, while in Examples 3-5, R1, R2 The reaction temperature and reaction results of R2 and R3 are shown in Table 1.
[0034] Table 1
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com