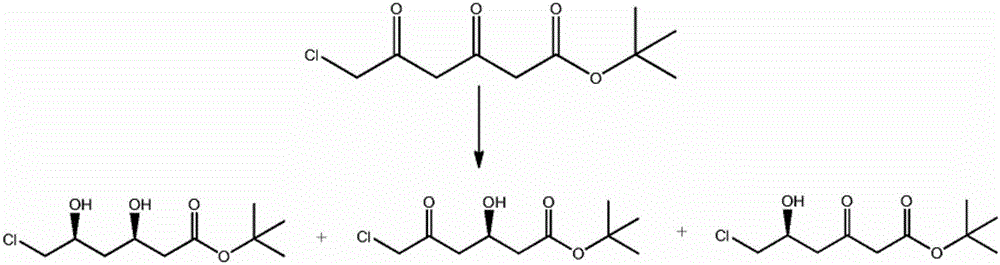
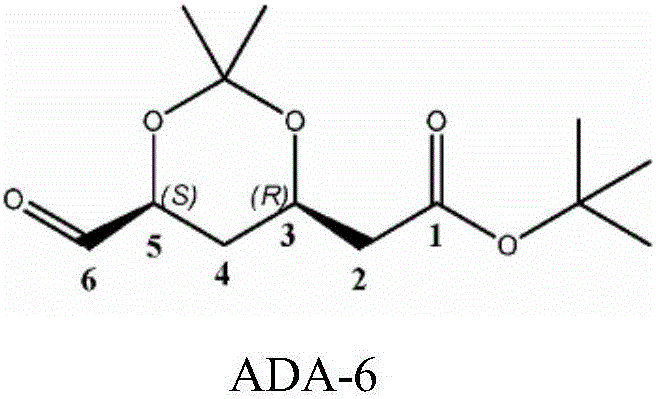
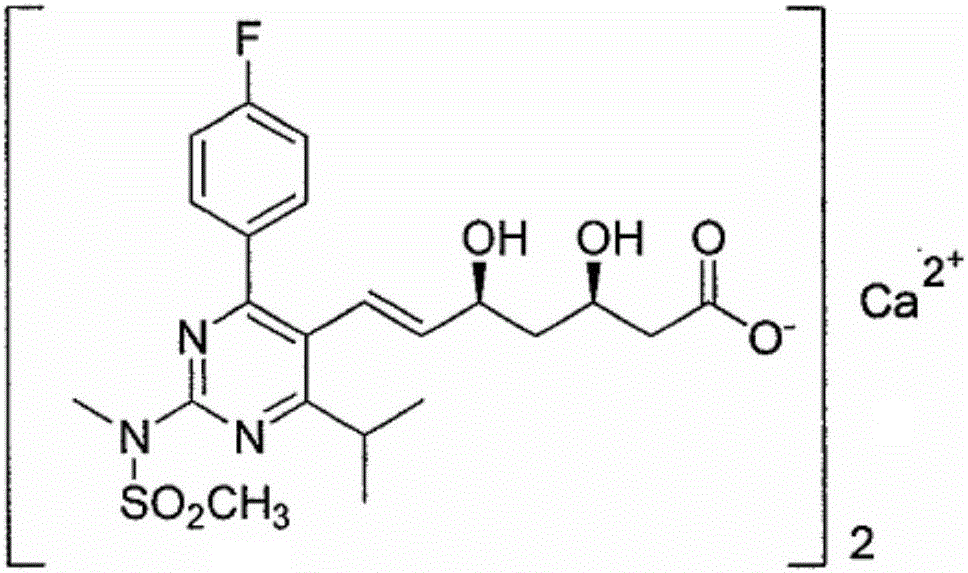
Method for synthesizing ADA
A synthetic method and intermediate technology, applied in the field of ADA synthesis, can solve the problems of low total yield, difficult industrial production, and difficult acquisition, and achieve cheap raw materials and reagents, high selectivity and yield, and simple operation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The synthetic method of ADA comprises following five steps:
[0083] Synthesis of intermediate (I)
[0084]
[0085] Add 150g of toluene and 300g of S-4-chloro-3-hydroxybutyronitrile in sequence to a 1L four-necked flask, stir at room temperature for 15min until dissolved, control the system temperature at 10-35°C, and add 306.25g of hexamethyldisilazane dropwise After 1 hour of dripping, the system was heated to reflux to react for 4 hours, and the residual S-4-chloro-3-hydroxybutyronitrile was detected by HPLC <0.5%, and the reaction was stopped; the oily intermediate 445.34g was obtained by drying under reduced pressure , the yield was 103.4%, and it was put into the next reaction without further purification.
[0086] Synthesis of intermediate (II)
[0087]
[0088] In a flask, dissolve 445.34g of intermediate I, 222.67g of zinc powder, and 9.90g of methanesulfonic acid in 1692g of tetrahydrofuran. After heating at 70°C for 1 hour, the system cools down to 0...
Embodiment 2
[0099] The synthetic method of ADA comprises following five steps:
[0100] Synthesis of compound (I)
[0101]
[0102] Add 150g of acetonitrile and 300g of S-4-chloro-3-hydroxybutyronitrile successively into a 1L four-neck flask, stir at room temperature for 15min to dissolve. Control the temperature of the system at 10-35°C, add 306.25 g of hexamethyldisilazane dropwise, and finish dropping within 1 hour. After dropping, the system was heated to reflux for 5 hours, and the reaction was stopped after HPLC detection of S-4-chloro-3-hydroxybutyronitrile remaining <0.5%. After drying under reduced pressure, 431.04 g of intermediate I was obtained as an oily intermediate, with a yield of 100.08%, which could be put into the next reaction without further purification.
[0103] Synthesis of compound (II)
[0104]
[0105] In a flask, dissolve 431.04g of intermediate I, 189.52g of iron powder, and 9.58g of methanesulfonic acid in 1638g of tetrahydrofuran. After heating at 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com