Nonionic functional fluorine-containing polymer and preparation method thereof
A non-ionic, functional technology, applied to the types of packaging items, special packaging items, medical containers, etc., can solve the problems of difficult control of content, limited application range, and failure of fluorine-containing copolymers to achieve hydrophilicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
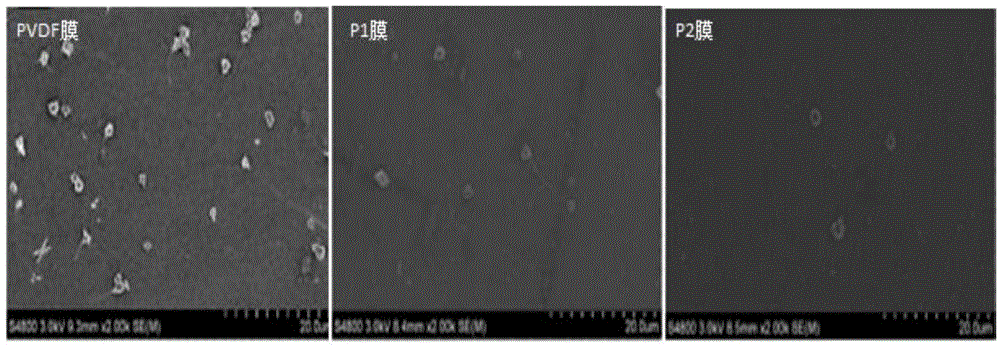
Image
Examples
Embodiment 1
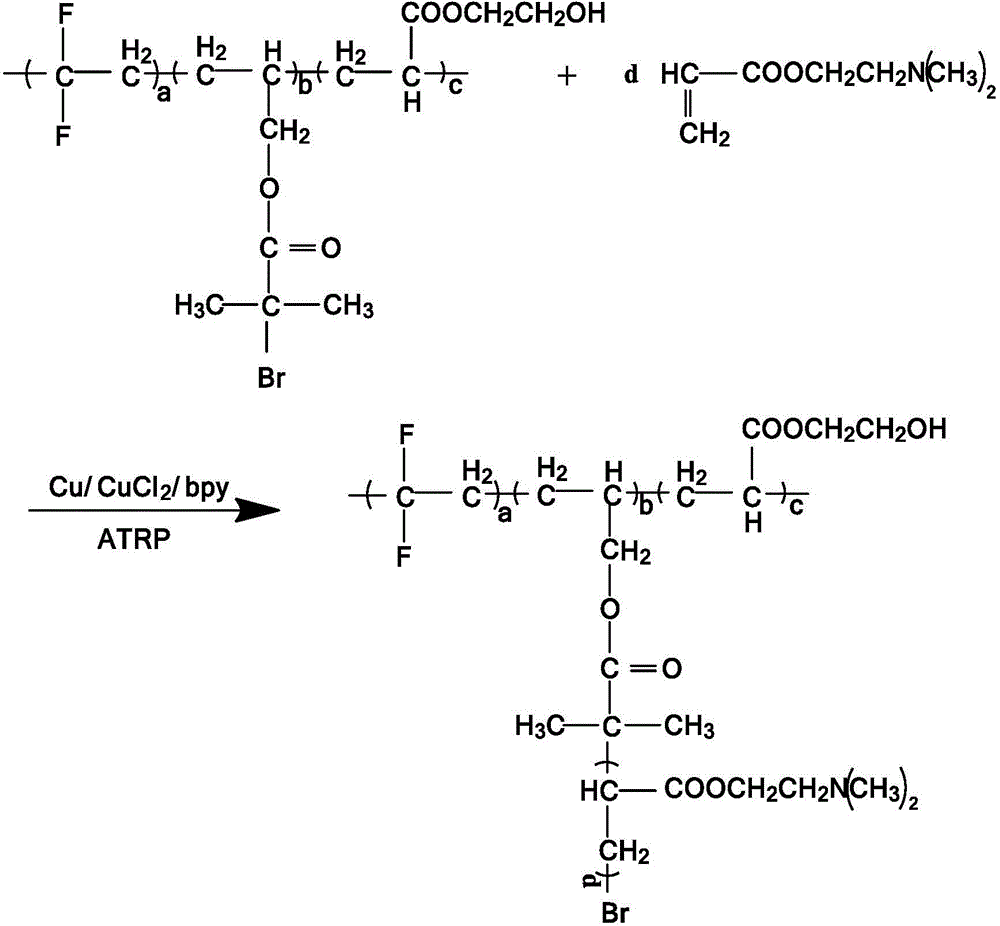
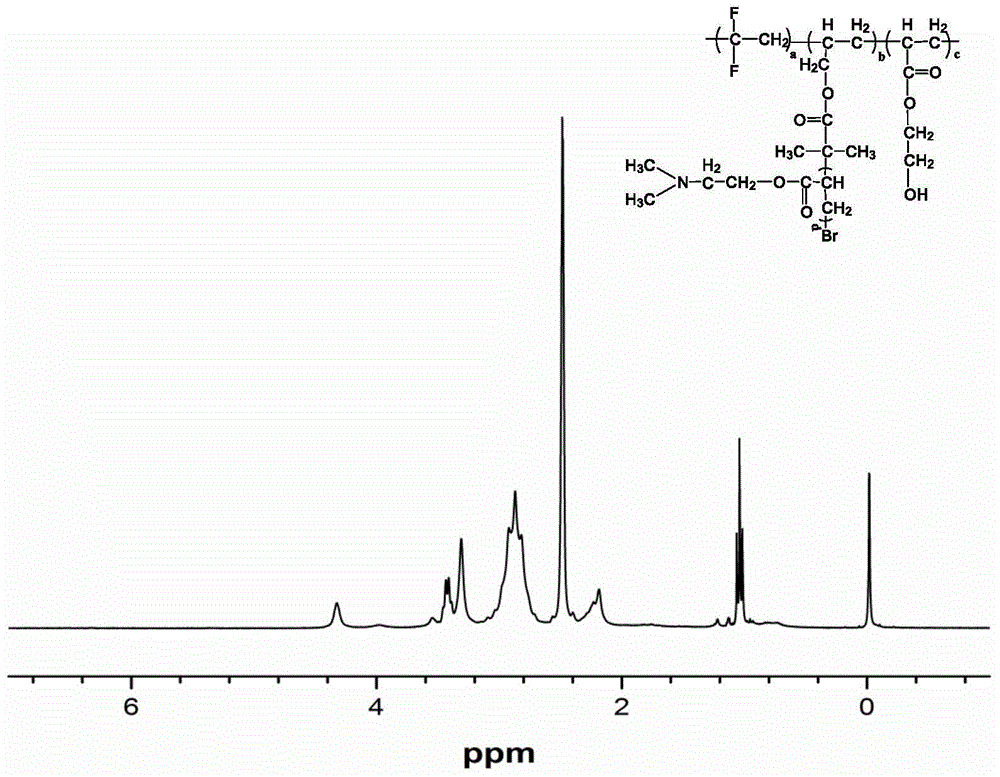
[0147] Synthesis of non-ionic functionalized fluoropolymer P1:
[0148] Add 2000ml deionized water, additive polyvinyl alcohol (PVA) 1.2g, additive hydroxypropyl methylcellulose (HPMC) 0.4g, initiator peroxydicarbonate bis(2-ethylhexyl) in the stainless steel reaction kettle (EHP) 1g, evacuated and filled with nitrogen repeatedly 3 times, then added 1500g of vinylidene fluoride, 27.19g of hydroxyethyl acrylate and 4.85g of allyl 2-bromo-2-methylpropionate, pre-dispersed and stirred at room temperature for 30 minute. The temperature was raised to a polymerization temperature of 47° C. to carry out a polymerization reaction. React for 12 hours, stop heating when the pressure drop in the kettle reaches 0.2MPa, volatilize naturally for 15 minutes, introduce air for 5 minutes, vacuumize and fill with nitrogen three times, then add 33.51g of dimethylaminoethyl acrylate, 5g of copper, chlorine Cuprous chloride 5g, 2,2'-bipyridine (bpy) 16g, then control the temperature at 60°C, and...
Embodiment 2
[0165] Synthesis of non-ionic functionalized fluoropolymer P2:
[0166] Referring to Example 1 for the synthesis process of P2, the formula and process parameters are shown in Table 1 and Table 2 respectively.
[0167] The P2 structure and performance characterization methods are the same as those in Example 1. The general formula of the P2 structural formula is the same as that of P1, wherein the parameters in the general formula are as shown in Table 3; the molecular weight and molecular weight distribution of P2 are as shown in Table 4; 5.
Embodiment 3
[0169] Synthesis of non-ionic functionalized fluoropolymer P3:
[0170] The synthesis process of P3 refers to Example 1, and the formula and process parameters are shown in Table 1 and Table 2 respectively.
[0171] The P3 structure and performance characterization methods are the same as those in Example 1. The general formula of the P3 structural formula is the same as that of P1, wherein the parameters in the general formula are shown in Table 3; the molecular weight and molecular weight distribution of P3 are shown in Table 4; 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com