Nickel composite hydroxide and manufacturing method thereof, cathode active material for nonaqueous-electrolyte secondary battery and manufacturing method thereof, and nonaqueous-electrolyte secondary battery
A composite hydroxide and cathode active material technology, applied in the direction of non-aqueous electrolyte batteries, active material electrodes, secondary batteries, etc., can solve the problems of undeveloped particle size uniformity, particle degradation, and no particle size, etc., to achieve Suitable for large-scale production and high industrial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
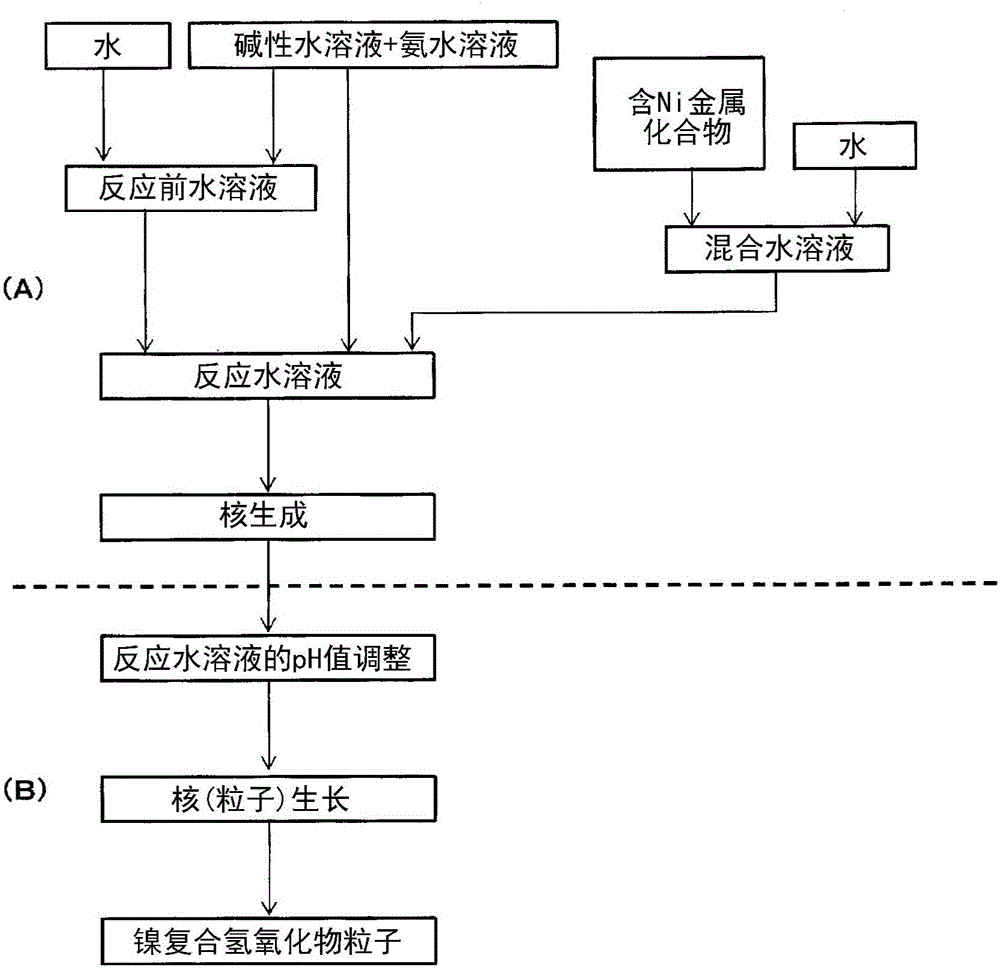
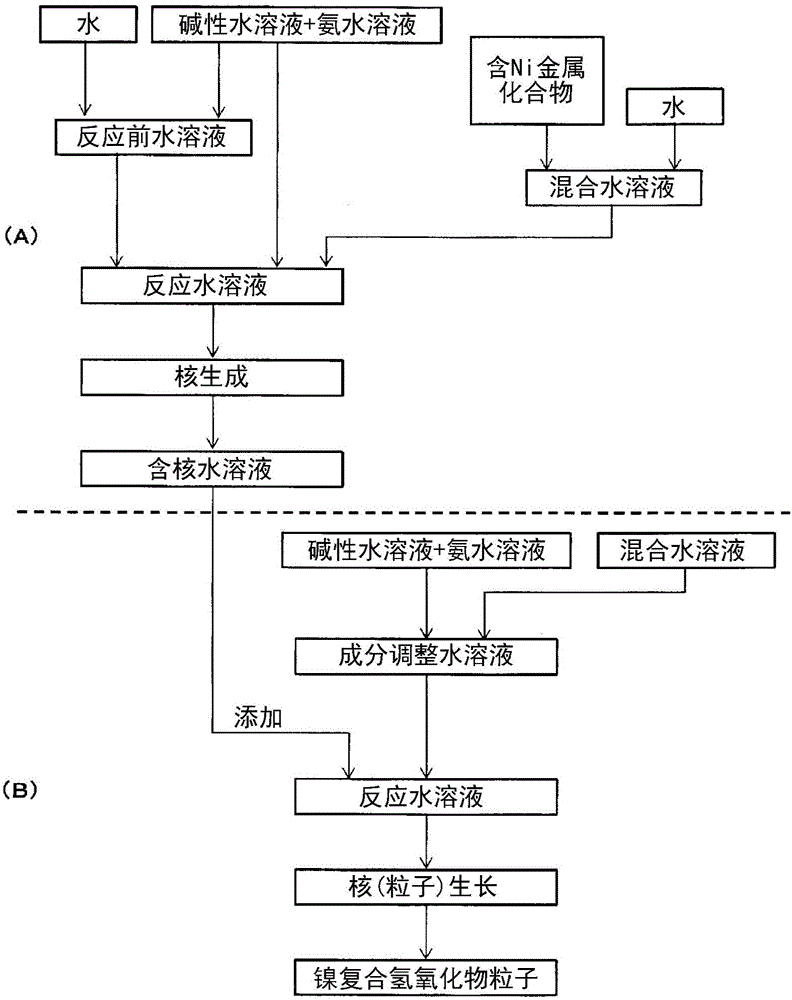
[0297] [Manufacture of nickel composite hydroxide]
[0298] (nucleation process)
[0299] Add 7.2 L of water to a baffled reaction tank with a capacity of 50 L capable of maintaining the environment in the tank, and flow nitrogen gas while stirring at 500 rpm with an inclined paddle-type stirring blade, thereby reducing the oxygen concentration in the environment in the reaction tank to less than 1% by volume, and adjusted the temperature in the tank to 40°C. Add an appropriate amount of 25% by mass sodium hydroxide aqueous solution and 25% by mass ammonia water to the reaction tank, adjust the pH value of the reaction solution in the tank under the liquid temperature standard of 25°C to be 12.6 and adjust the ammonia concentration to be 10g / L, thus forming a reaction pre-water solution.
[0300]Next, 35 mL of a mixed aqueous solution of 1.9 mol / L obtained by dissolving nickel sulfate and manganese sulfate (the molar ratio of metal elements being Ni:Mn=50:50) in water was ad...
Embodiment 2
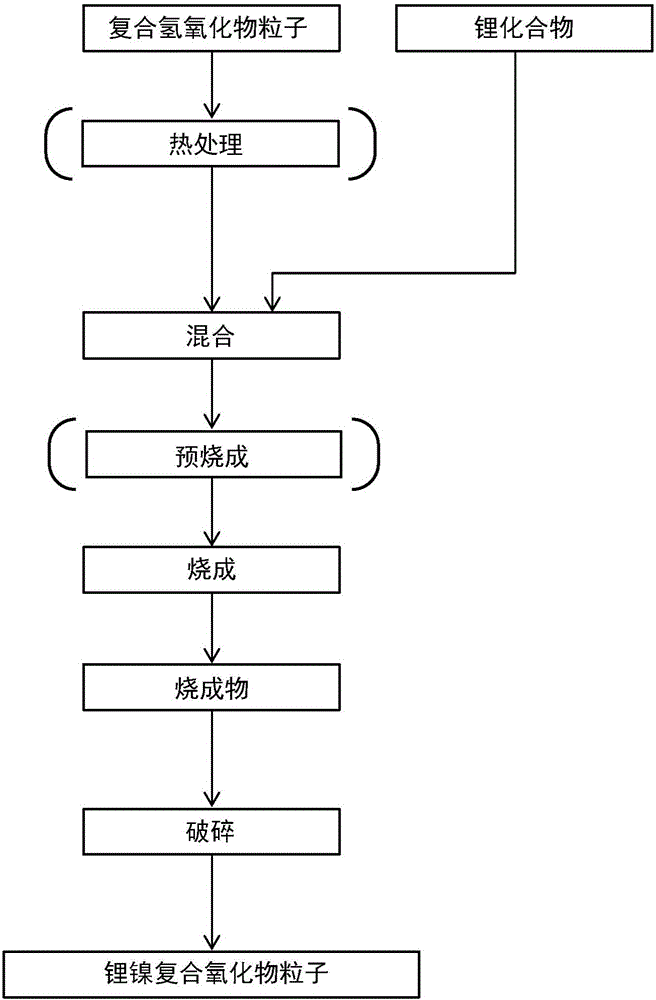
[0326] In the composite hydroxide manufacturing process, in addition to nickel sulfate and manganese sulfate, sodium tungstate is also dissolved in water to form a mixed aqueous solution, except that it is operated in the same manner as in Example 1 to obtain a non-aqueous electrolyte secondary battery A positive electrode active material was used and evaluated. In addition, in this mixed aqueous solution, the molar ratio of each metal element was adjusted to Ni:Mn:W=49.75:49.75:0.5. The composition of the obtained composite hydroxide is Ni 0.4975 mn 0.4975 W 0.005 (OH) 2+a (0≤a≤0.5). In addition, the composition of the obtained positive electrode active material is Li 1.20 Ni 0.4975 mn 0.4975 W 0.005 o 2 , It was confirmed by powder X-ray diffraction that it is composed of a single phase of hexagonal layered crystal lithium-nickel-manganese composite oxide.
Embodiment 3
[0328] In the composite hydroxide production process, in addition to nickel sulfate and manganese sulfate, zirconium sulfate was also dissolved in water to form a mixed aqueous solution, except that it was performed in the same manner as in Example 1 to obtain a non-aqueous electrolyte secondary battery. A positive electrode active material was used and evaluated. In addition, in this mixed aqueous solution, the molar ratio of each metal element was adjusted to Ni:Mn:Zr=49.75:49.75:0.5. The composition of the obtained hydroxide is Ni 0.4975 mn 0.4975 Zr 0.005 (OH) 2+a (0≤a≤0.5). In addition, the composition of the obtained positive electrode active material is Li 1.20 Ni 0.4975 mn 0.4975 Zr 0.005 o 2, It was confirmed by powder X-ray diffraction that it is composed of a single phase of hexagonal layered crystal lithium-nickel-manganese composite oxide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com