Cationic lipid compound as well as preparation method and application thereof
A technology of cationic lipids and compounds, applied in the direction of steroids, chemical instruments and methods, and other methods of inserting foreign genetic materials, can solve the problems of high cytotoxicity, high price, and low cell transfection rate, and achieve transfection Strong dyeing ability, high biocompatibility, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
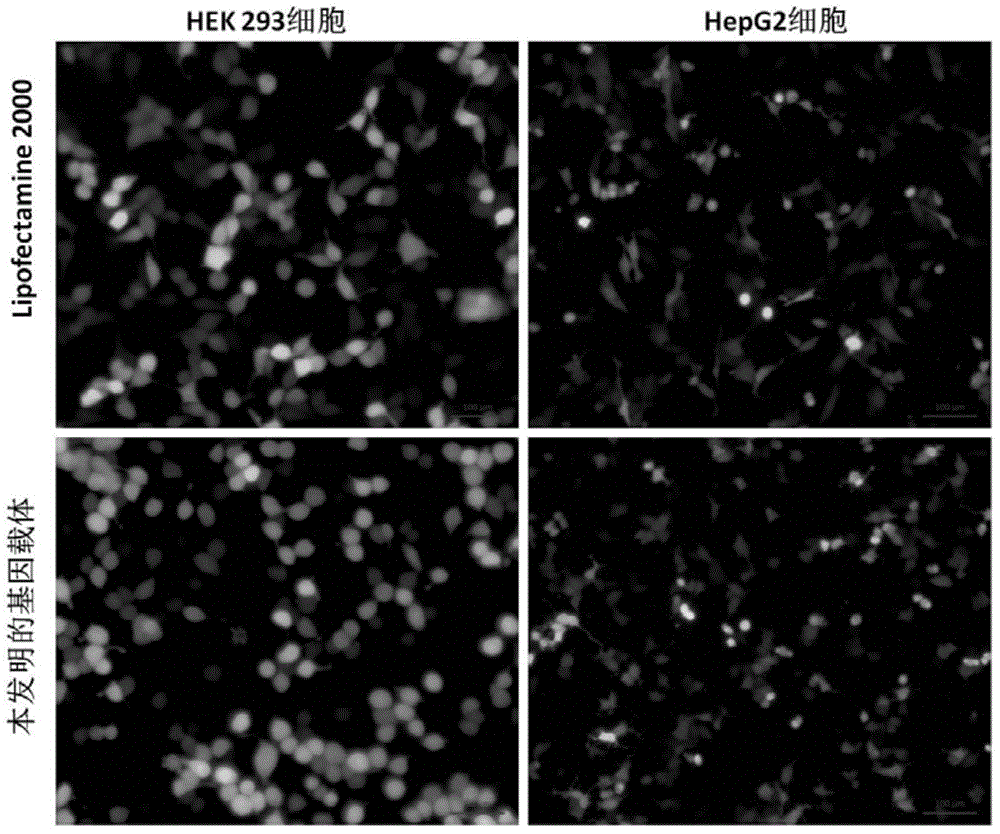
Examples
Embodiment 1
[0060] The synthesis of embodiment 1 raw material
[0061] Synthesis of compound I (saturated / unsaturated aliphatic hydrocarbon amide of glutamic acid): dissolve saturated / unsaturated hydrocarbon-based ammonia or alcohol, Boc-glutamic acid-OH in a dry organic solvent, and add a condensing agent under nitrogen protection , after stirring at 0-35°C for 12-24 hours, distill off the organic solvent, add excess acid mixture, stir at room temperature for 1-2 hours, distill off the solvent, and use silica gel column chromatography to separate and purify the intermediate glutamic acid Saturated / unsaturated hydrocarbyl substituted esters or amides with a yield of 70%.
[0062] Synthesis of compound II (saturated / unsaturated aliphatic hydrocarbon amide of aspartic acid): dissolve saturated / unsaturated hydrocarbon-based ammonia or alcohol, Boc-aspartic acid-OH in a dry organic solvent, add under nitrogen protection Condensing agent, stir at 0-35°C for 12-24 hours, distill off the organi...
Embodiment 2
[0063] The synthesis of embodiment 2 raw materials
[0064] Synthesis of Compound III (Boc-protected peptidomimetics):
[0065] The first step: Stir Boc-ornithine and Boc-ornithine (Boc)-OSu in an organic solvent under alkaline conditions at 0-35°C for 24-36 hours, distill off the organic solvent and use silica gel column chromatography The intermediate Boc-ornithine (Boc)-ornithine (Boc)-COOH is obtained by separation and purification by the method, and the Boc is di-tert-butyl carbonate. Yield: 60%.
[0066] The second step: Stir the intermediate, N-hydroxysuccinimide, and condensing agent obtained in the first step in an organic solvent for 12 hours, then add Boc-ornithine, and continue stirring at 0-35°C for 12-24 hours After the organic solvent was distilled off, the compound II, Boc-Ornithine (Boc)-Ornithine (Boc)-Ornithine (Boc)-COOH, was obtained by separation and purification by silica gel column chromatography. The Boc is di-tert-butyl carbonate. Yield: 65%.
[...
Embodiment 3
[0074] Embodiment 3 The preparation of compound of the present invention (1)
[0075]
[0076] Raw materials include compound I (glutamic acid fatty alkane amide molecule), compound III (amino Boc-protected short peptide molecule), condensation agent (1-ethyl-(3-dimethylaminopropyl) carbodiimide salt Acid EDCI), base (N,N-diisopropylethylamine DIPEA) dissolved in dry dichloromethane, compound I: compound III: condensing agent: base (molar ratio) = 1:1:2:2 , The amount of solvent is limited to the complete dissolution of compound I, compound III, condensing agent and base.
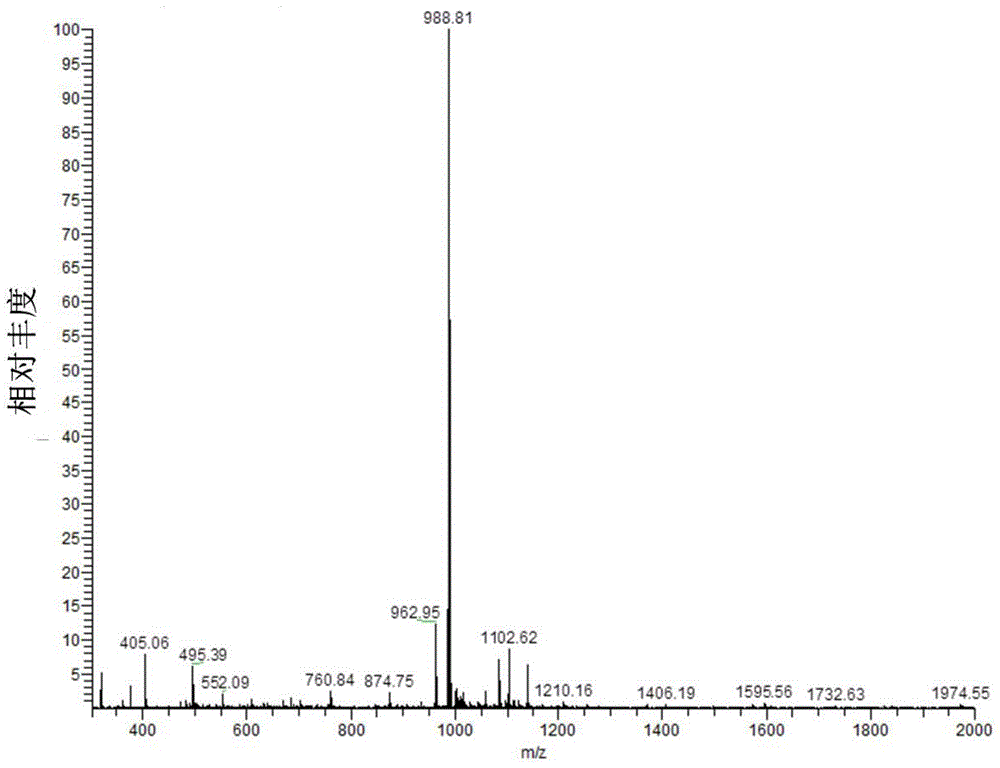
[0077] Under the protection of nitrogen, the mixture was cooled to 0°C with an ice bath and then stirred at 25°C for 24 hours. After the reaction was over, the reaction mixture was dissolved in dichloromethane, washed 3 times with 5% citric acid solution, water, and saturated brine successively, dried with anhydrous sodium sulfate, and then purified by silica gel column chromatography (eluent: dichlorom...
PUM
| Property | Measurement | Unit |
|---|---|---|
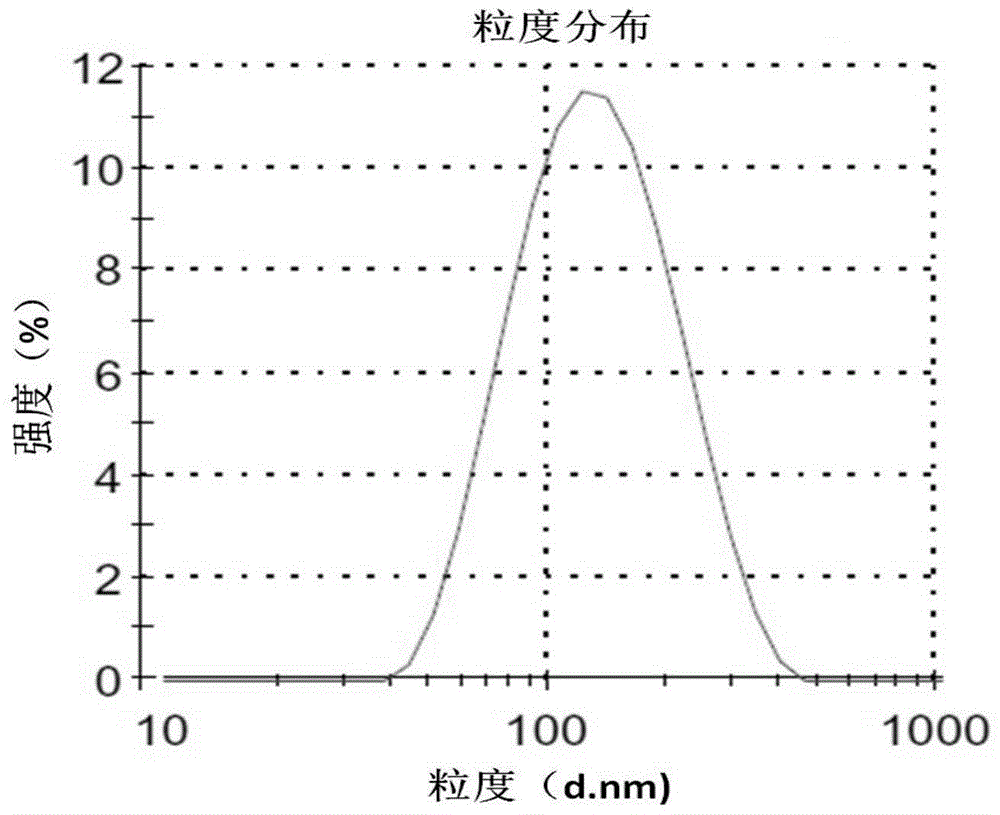
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com