A kind of preparation method of epoxiconazole intermediate and the preparation method of epoxiconazole
A technology of epoxiconazole and intermediates, which is applied in the preparation of epoxiconazole intermediates and the preparation of epoxiconazole, can solve the problems of high water and oxygen content requirements, safety and environmental protection problems, and unfavorable large-scale production. The effect of simple operation, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
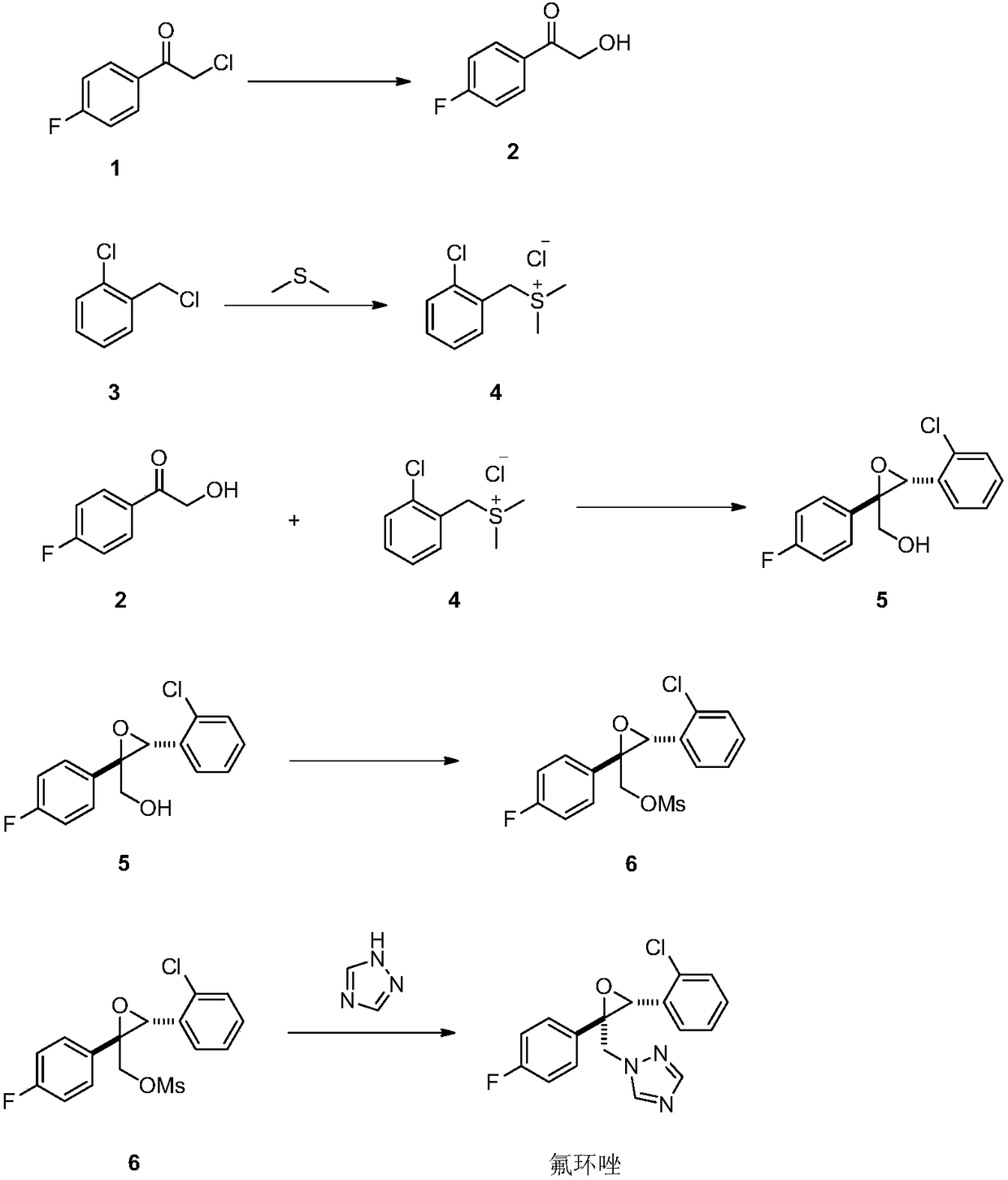
[0033] Dissolve 25 grams of compound 1 in 50 mL of dimethyl sulfoxide, add 15 grams of sodium hydroxide and 1 gram of potassium iodide, heat up to 100 ° C, and react for 5 hours. When there is no compound 1 detected by GC, stop heating, cool to room temperature, and add acetic acid. The ethyl ester was washed with water, 5% sodium chloride solution and water in turn, separated, dried, and distilled under reduced pressure to remove ethyl acetate to obtain 21 g of compound 2, with a purity of >95% and a yield of 95.5%.
Embodiment 2
[0035] Dissolve 25 g of o-chlorobenzyl chloride in 50 mL of dimethyl sulfide, heat to 40 ° C, react at this temperature for 3 hours, stop heating, cool to 5 ° C, add 50 mL of toluene and 21 g of compound 2 obtained in Example 1 , then added potassium hydroxide in batches, heated to 80 ° C, collected the remaining dimethyl sulfide in the system, reacted for 2 hours, when GC could not detect compound 1, cooled to room temperature, added 50 mL of water, layered, and the organic layer was successively watered , washed with 5% sodium chloride solution and water, dried, and distilled under reduced pressure to obtain 40 g of compound 5 with a purity of >92% and a yield of 93%.
Embodiment 3
[0037] Dissolve 25 g of o-chlorobenzyl chloride in 50 mL of dimethyl sulfoxide, heat to 50 °C, react at this temperature for 3 hours, stop heating, cool to 5 °C, add 21 g of compound 2 obtained in Example 1, and then divide Sodium amide was added in batches, heated to 80°C, and reacted for 2 hours. When no compound 1 was detected by GC, it was cooled to room temperature, 50 mL of water was added, and the layers were separated. The organic layer was washed successively with water, 5% sodium chloride solution and water, and dried. Distillation under reduced pressure gave 35 g of compound 5 with a purity of >91% and a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com