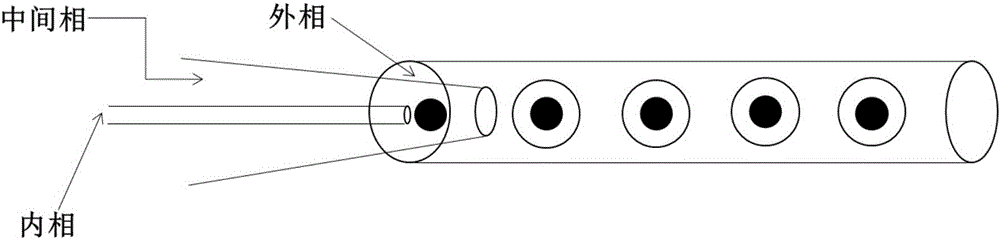
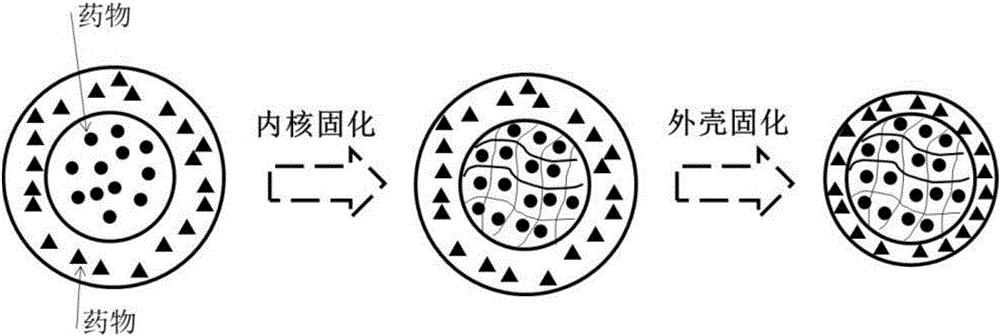
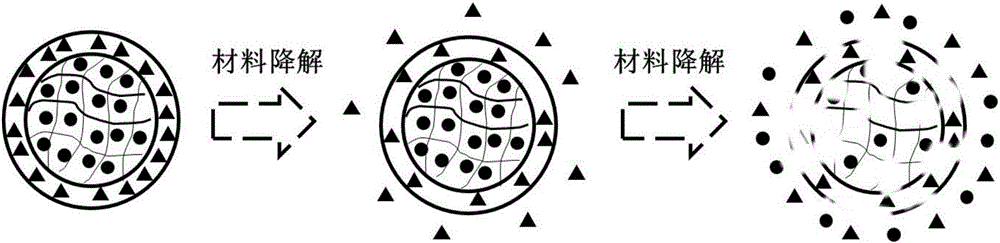
Compound medicine microcarrier with core-shell structure
A technology of core-shell structure and microcarriers, which is applied in drug delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the difficulties of co-delivery, the limitation of sustained release effect, and the size distribution of microcarriers. Large and other problems, to achieve the effect of improving encapsulation efficiency and drug loading, good biocompatibility, and a wide range of options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 gelatin-methyl methacrylate-polylactic acid glycolic acid (DOX-CPT-GelMa-PLGA) core-shell structure microcarrier loaded with doxorubicin-camptothecin:
[0031] 1. Fabrication of double emulsion microfluidic chip: use acetylene blowtorch to draw glass capillary, then grind the nozzle to the required size with sandpaper, ultrasonically clean in alcohol, and use 3-aminopropyl tris A 2%-10% ethanol solution of ethoxysilane (APTES) is used for hydrophilic treatment; the inner phase gelatin-methyl methacrylate capillary is made hydrophobic with a 2%-10% solution of octadecyltrimethoxysilane in acetone deal with. Glass capillary microfluidic chips were assembled with hydrophilic and hydrophobic treated glass capillaries, glass slides, coverslips, sampling needles and quick-drying glue.
[0032] 2. Preparation of double-emulsion template: prepare each phase solution, dissolve 0.5mg DOX in 1mL 15% GelMa aqueous solution as the inner water phase, ...
Embodiment 2
[0035] The preparation of embodiment 2 methyl methacrylate-polylactic acid glycolic acid-gelatin-methyl methacrylate (CPT-DOX-PLGA-GelMa) core-shell structure microcarrier loaded with camptothecin-doxorubicin:
[0036] 1. Fabrication of double-emulsion microfluidic chips: Use an acetylene blowtorch to draw a glass capillary, then grind the nozzle to the required size with sandpaper, clean it ultrasonically in alcohol, and use 18 2%-10% acetone solution of alkyltrimethoxysilane is used for hydrophobic treatment, and the inner phase is injected into the glass capillary of oil phase PLGA solution using 2%-10% ethanol solution of 3-aminopropyltriethoxysilane (APTES) Hydrophilic treatment;. Glass capillary microfluidic chips were assembled with hydrophilic and hydrophobic treated glass capillaries, glass slides, coverslips, sampling needles and quick-drying glue.
[0037] 2. Preparation of double-emulsion template: prepare each phase solution, dissolve 1g PLGA in 10mL dichlorometh...
Embodiment 3
[0039] Example 3 Glycidyl methacrylate chitosan-polylactic acid (5-Fu-PTX-DexGMa-PLLA) core-shell structure microcapsules in vitro drug release loaded with 5-fluorouracil-paclitaxel:
[0040]1. Fabrication of double-emulsion microfluidic chips: use acetylene blowtorch to draw glass capillary, then grind the nozzle to the required size with sandpaper, ultrasonically clean in alcohol, and use 3-aminopropyl tris A 2%-10% ethanol solution of ethoxysilane (APTES) was used for hydrophilic treatment; a capillary injected into Dex-GMa was treated with a 2%-10% acetone solution of octadecyltrimethoxysilane for hydrophobic treatment. Glass capillary microfluidic chips were assembled with hydrophilic and hydrophobic treated glass capillaries, glass slides, coverslips, sampling needles and quick-drying glue.
[0041] 2. Preparation of double-emulsion template: Prepare each phase solution, dissolve 0.5mg 5-Fu in 1mL 15% DexGMa aqueous solution as the inner aqueous phase, dissolve 1g PLLA i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com