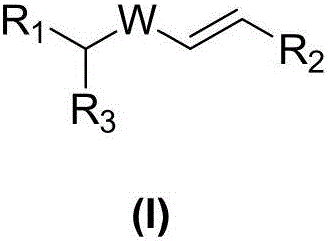
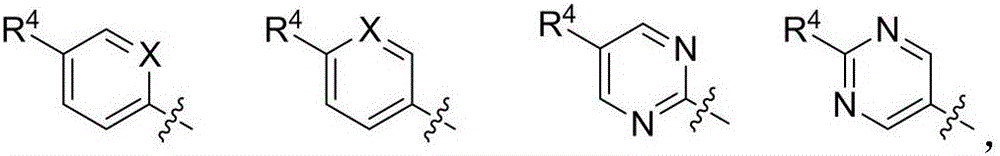
New compound with radiation protection function, preparation method and pharmaceutical application thereof
A compound and solvate technology, used in the field of medicine, can solve the problems of limiting the wide application of radiotherapy, short half-life, high price, etc., and achieve the effects of prolonging the survival period and survival rate of animals, alleviating the side effects of radiotherapy, and reducing biological damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] The preparation method of general formula (I) compound:
[0099]
[0100] The preparation methods of compounds (II) and (III) are carried out with reference to the synthesis method of compound (I).
Embodiment 1
[0101] Example 1: Synthesis of 4-(2-(((5-chloropyridin-2-yl)methyl)sulfonyl)vinylbenzoic acid (Compound 1)
[0102] Step 1: Preparation of 2–(((5-chloropyridin-2-yl)methyl)thio)acetic acid
[0103]
[0104] Put the calculated NaOH solid (2.7g, 66.9mmol, easy to absorb moisture, pay attention) into the round bottom flask, add water, stir to dissolve, cool to room temperature, slowly add thioglycolic acid (3.1g, 33.4mmol ), after a few minutes of reaction, add p-5-chloro-2-(chloromethyl)pyridine (5.0g, 31.1mmol), react at 50°C, the oil layer gradually disappears and the solution becomes homogeneous, TLC monitors the reaction, and stops the reaction after about 2h , under cooling in an ice bath, adjust the acidity with hydrochloric acid, and the pH value is about 3. An appropriate amount of crushed ice was added, and a white solid was precipitated, filtered with suction, washed twice with cold water, and left to air to obtain a white solid. Yield: 73.58%. 1 H NMR (400MHz, C...
Embodiment 2
[0111] Example 2: Synthesis of 4-(2-(((5-chloropyridin-2-yl)methyl)sulfonyl)vinylbenzoic acid (Compound 2)
[0112] Step 1: Preparation of 2–((4-chlorobenzyl-2-yl)mercapto))acetic acid
[0113]
[0114] Put the calculated NaOH solid (2.7g, 66.9mmol, easy to absorb moisture, pay attention) into the round bottom flask, add water, stir to dissolve, cool to room temperature, slowly add thioglycolic acid (3.1g, 33.4mmol ), add p-chlorobenzyl chloride (5.0g, 31.1mmol) after a few minutes of reaction, react at 50°C, the oil layer gradually disappears and the solution becomes homogeneous, TLC monitors the reaction, stops the reaction after about 2.5h, cools in an ice bath, and uses Hydrochloric acid to adjust the acidity, the pH value is about 3. An appropriate amount of crushed ice was added, and a white solid was precipitated, filtered with suction, washed twice with cold water, and left to air to obtain a white solid. Yield: 73.4%. 1 H NMR (400MHz, CDCl 3 ): δ13.05(s,1H),7.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com