Synthetic method and application of metal-organic framework composite nanomaterial
A nano-composite material and metal-organic framework technology, which is applied in the direction of alkali metal compounds, analytical materials, and material inspection products, can solve the problems of low abundance of glycopeptides and phosphorylated peptides, low ionization efficiency, and difficulties in mass spectrometry detection. Good body pore structure, improved mass spectrometry signal, high sensitivity and selectivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
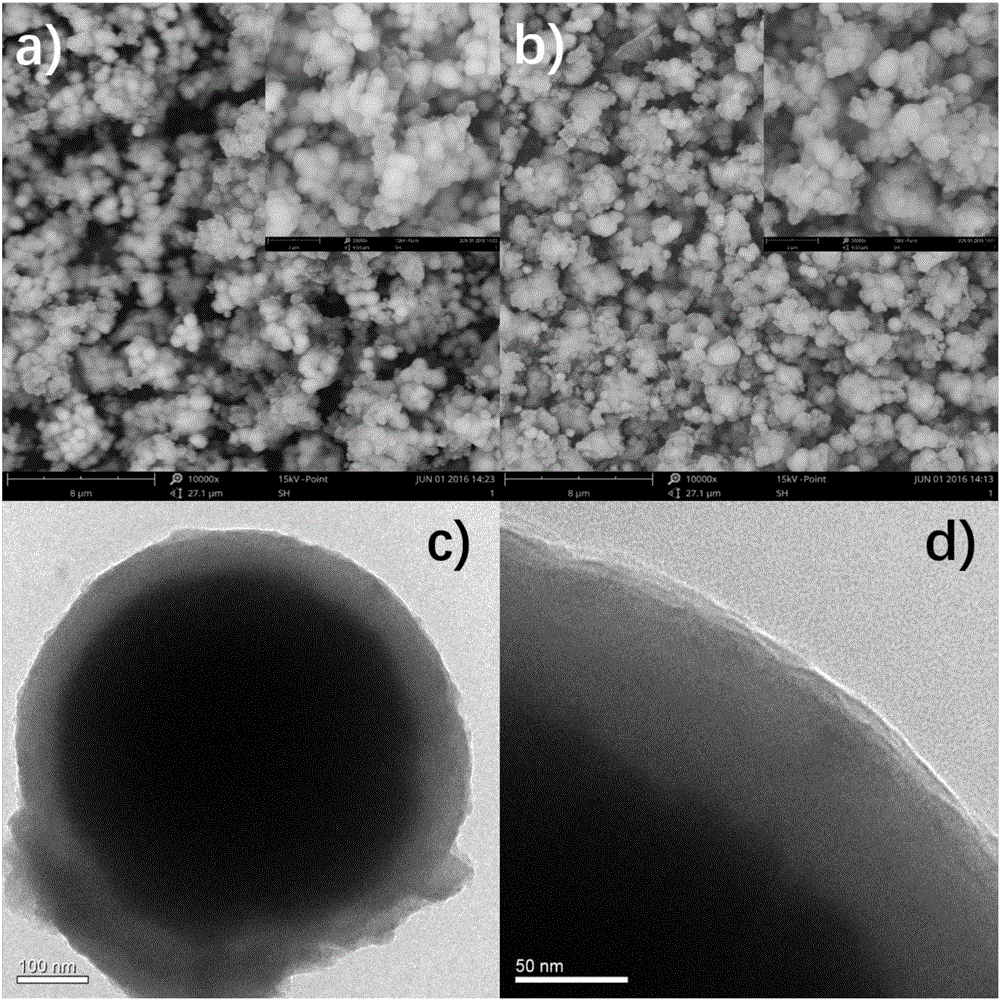
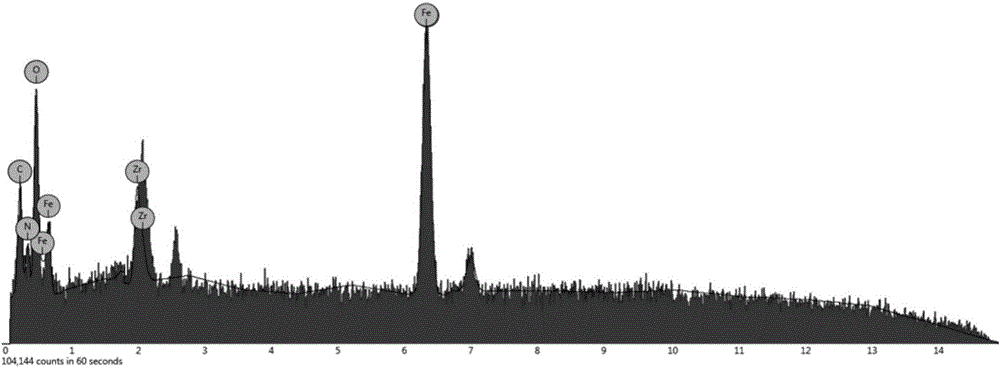
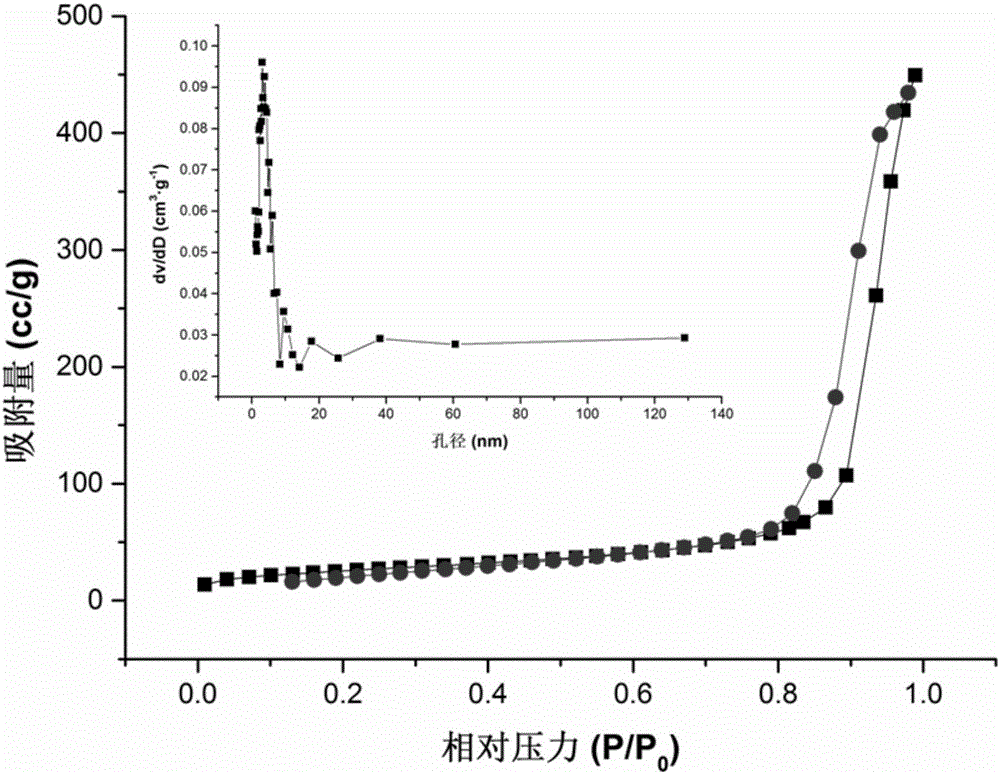
[0042] Example 1: Synthesis of a metal-organic framework (MOF) nanocomposite material with zirconium as the central metal ion, coated with polydopamine and amino groups on the surface of magnetic balls.
[0043] (1) Using ethylene glycol as a solvent to synthesize ferroferric oxide magnetic balls, 1.35g FeCl 3 •6H 2 O was dissolved in 75mL of ethylene glycol, stirred magnetically (sealed with gloves) until clear, then added 3.6g of crushed sodium acetate and stirred until dissolved and continued stirring for 0.5h (sealed with gloves). After ultrasonication for 5 minutes, transfer to the reaction kettle, 200°C, 16h. The reaction kettle was taken out and cooled overnight. Pour out the magnetic ball and wash it with water 5 times (sonication for 5 minutes each time). Fully wash the magnetic balls with deionized water and ethanol until the washing solution is clear and pure, and dry in vacuum at 50°C;
[0044] (2) Prepare Tris (Tris) buffer solution (solvent is deionized water...
Embodiment 2
[0057] Example 2: The surface of the magnetic ball obtained in Example 1 is coated with polydopamine and amino-modified metal-organic framework (MOF) nanocomposites with zirconium as the central metal ion as a solid-phase microextraction adsorption separation medium for low-concentration HRP The enrichment and MALDI-TOF MS detection of enzymatic hydrolyzate and β-Casein enzymolyzate.
[0058] (1) Preparation of standard protein enzymatic hydrolysis solution: Accurately weigh 2 mg of HRP standard protein, use 25 mM ammonium bicarbonate solution to prepare a standard protein solution with a concentration of 2 mg / mL, the pH is about 8.3, and boil for ten minutes. According to the ratio of trypsin to standard protein with a mass ratio of 1:50, add trypsin and incubate at 37°C for 16 hours to obtain 2 mg / mL HRP trypsin hydrolyzate; accurately weigh 4 mg IgG standard For protein, use 25 mM ammonium bicarbonate solution to make a standard protein solution with a concentration of 4 mg...
Embodiment 3
[0067] Example 3: The surface of the magnetic balls obtained in Example 1 is coated with polydopamine and amino-modified metal-organic framework (MOF) nanocomposites with zirconium as the central metal ion as a solid-phase microextraction adsorption separation medium for HRP enzymolysis Enzyme solution or β-Casein enzymolysis solution and bovine serum albumin (BSA) enzymolysis solution enrichment and MALDI-TOF MS detection.
[0068] (1) Preparation of standard protein hydrolyzate: Accurately weigh 2 mg of standard protein HRP, 2.5 mg of standard protein β-Casein and 5 mg of standard protein BSA, and use 25 mM ammonium bicarbonate solution to make a concentration of 2 mg / mL, 2.5 mg / mL and 5 mg / mL standard protein solutions, pH about 8.3, boiled for 10 minutes. According to the ratio of trypsin to standard protein at a mass ratio of 1:50, add trypsin and incubate at 37°C for 16 hours to obtain 2 mg / mL HRP trypsin hydrolyzate and 2.5 mg / mL β- Casein enzymatic hydrolysis solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com