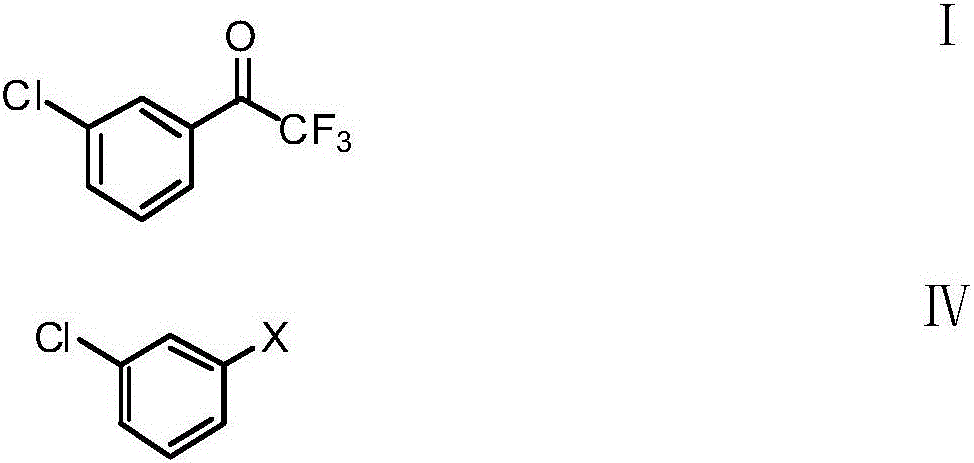Method for preparing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate and free alkalis thereof
A technology of trifluoroacetate and compound, which is applied in the field of chemistry and pharmacy, and can solve the problems of expensive raw materials, many raw materials, and expensive prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] In a preferred embodiment of the present invention, the preparation method of 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate and its free base comprises steps:
[0102] In the first step, adopt the method provided by the present invention to obtain 2,2,2-trifluoro-(3'-chlorophenyl)ethanone; for example, m-chlorobromobenzene (3-chlorobromobenzene), m-chloroiodine Benzene (3-chloroiodobenzene) or m-dichlorobenzene is dissolved in tetrahydrofuran, methyl tert-butyl tert-butyl ether, petroleum ether and other ether solvents, or dissolved in benzene, toluene, pentane, hexane, heptane and other solvents , or dissolved in a mixed solvent of these solvents, and obtain an intermediate by halogen-metal exchange with butyllithium or isopropylmagnesium chloride lithium chloride at low temperature, and then react with trifluoroacetate or trifluoroacetate metal salt , adding acid (including hydrochloric acid, sulfuric acid, phosphoric acid); preferably hydrochloric acid) a...
Embodiment 1
[0112] Preparation of 2,2,2-trifluoro-(3'-chlorophenyl)ethanone
[0113] Accurately weigh m-chlorobromobenzene (3-chlorobromobenzene) (19.15 g, 0.1 mol) and dissolve it in 100 ml of tetrahydrofuran, cool the solution in an ethanol dry ice bath to minus 78 to minus 50°C, and add butyllithium dropwise solution (0.12mol), after the dropwise addition was completed, it was kept at minus 78 to minus 50°C for 1 hour, and then ethyl trifluoroacetate (18.4 g, 0.13mol) was added dropwise at this temperature, and the cold bath was removed after the dropwise addition was completed. Naturally raised to room temperature, then hydrochloric acid (30ml, 0.3mol) was added dropwise, stirred and separated, and the organic layer was desolvated under reduced pressure to obtain crude product 2,2,2-trifluoro-(3'-chlorophenyl)ethanone 20.7 gram, distilled to obtain 20.3 grams of colorless transparent liquid, content 95%, yield 97.3%
[0114] GC-MS: 208.6. H-NMR: 7.655-7.687(t, 1H); 7.854-7.875(m, 1H...
Embodiment 2
[0116] Preparation of 2,2,2-trifluoro-(3'-chlorophenyl)ethanone
[0117] Accurately weigh m-chloroiodobenzene (3-chloroiodobenzene) (23.85 grams, 0.1mol) and dissolve it in 120 ml of methyl tert-butyl ether, and cool the solution in an ethanol dry ice bath to minus 20 to minus 10°C. Add dropwise isopropylmagnesium chloride solution (0.13mol), after the dropwise addition is completed, keep warm between minus 20 and minus 10°C for 1 hour, then add ethyl trifluoroacetate (18.4 grams, 0.13mol) dropwise at this temperature, dropwise After completion, remove the cold bath and stir to rise to normal temperature naturally, then add hydrochloric acid (30ml, 0.3mol) dropwise, stir and separate layers, and the organic layer is decompressed to remove the solvent to obtain 2,2,2-trifluoro-(3'-chlorobenzene Base) 20.5 grams of ethyl ketone crude product, distill to obtain 18.3 grams of colorless transparent liquid, content 95.9.7%, yield 88%
[0118] GC-MS: 208.6. H-NMR: 7.655-7.687(t, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com