Metal complex, preparation method thereof and preparation method of olefin binary copolymer
A technology of olefin binary copolymer and metal complex, applied in the fields of materials and chemical industry, can solve the problems of lack of polymer and insufficient catalytic efficiency, and achieve the effect of excellent mechanical properties and thermal properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
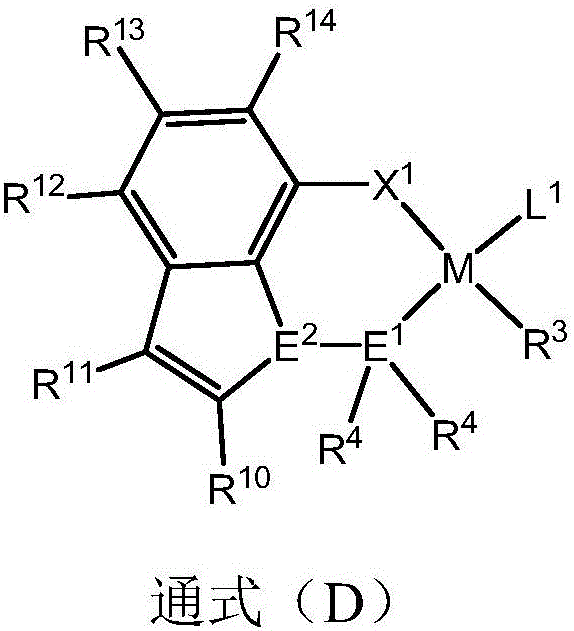
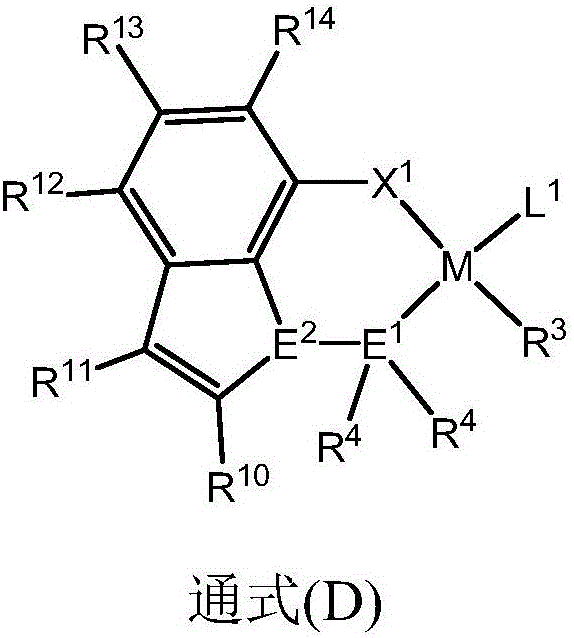
[0034] The preparation method provided by the present invention is to use a transition metal complex with a ligand of a specific structure as a catalyst component for α-olefin, acrylate, methacrylate, acrylic acid, unsaturated carboxylate, acrylamide, Acrylonitrile, unsaturated enol / ether, norbornene carboxylate and other monomers are copolymerized to obtain a polymer with a higher molecular weight, and the molecular chain structure is a binary copolymer. The ligand with a specific structure is a ligand that uses an aromatic heterocycle as a skeleton and is combined with two or more heteroatoms such as nitrogen, phosphorus, arsenic or antimony.
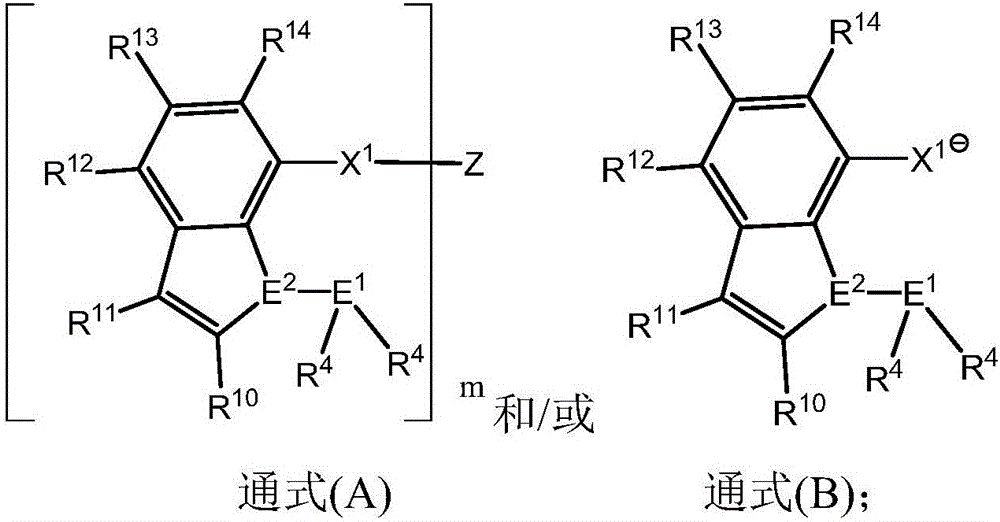
[0035] The present invention provides by reacting specific compounds (A) and / or (B) with transition metal complexes (C) including transition metals belonging to Group IVB to Group VIIB or Group VIII in the periodic table of elements such as nickel and palladium. The metal complex (D) is obtained. And it is provided that by making the...
Embodiment
[0128] The present invention is explained in detail in the following Examples and Comparative Examples, however, the present invention is not limited thereto.
[0129] In the following synthesis examples, unless otherwise stated, operations were performed under a purified nitrogen atmosphere, and dehydrated and deoxygenated solvents were used.
[0130] 1. Evaluation method
[0131] (1) Tm and Tc: Determined by the following DSC measurement.
[0132] Using a PYRIS Diamond DSC differential scanning calorimeter (PerkinElmer Inc.), sample 1 (approximately 5 mg) was melted at 210°C for 5 minutes, and then the temperature was decreased to -20°C at a rate of 10°C / min. After 5 minutes at -20°C, the temperature was raised to 210°C at a rate of 10°C / min to obtain a melting curve. The highest temperature of the main exothermic peak in the temperature lowering step was defined as the crystallization temperature Tc. In addition, the peak top temperature of the main endothermic peak in t...
Embodiment 1
[0135] Example 1: Synthesis of Ligand L1
[0136]
[0137] Add 1a (446mg, 2mmol) sequentially to a 50ml Schlenk bottle that was dried and replaced with nitrogen atmosphere, vacuumize and replace with nitrogen three times, add 15mL freshly distilled diethyl ether into the syringe, and add n-butyllithium dropwise at -5°C Solution (2.4mmol, 2.3M, 1mL), the resulting reaction system rose to room temperature and reacted for 2h. Diphenylphosphine chloride 1c (0.5 g) was added dropwise at 0° C., and the resulting reaction solution was warmed to room temperature. The initially obtained clear solution gradually became cloudy. After 2 hours, the reaction stopped and a white solid precipitated out. Post-processing: It was detected by TLC spotting that the raw materials were basically completely consumed, and the organic phase was spin-dried and purified by column chromatography to obtain 420 mg of the product (52%).
[0138]
[0139] Dissolve 407 mg of the above-mentioned raw mate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com