Nano-fibers with drug two-grade pulse releasing function and preparation method thereof
A pulse release and nanofiber technology, applied in the field of materials science, can solve the problem that nanofibers cannot effectively control the secondary pulse controlled release of drugs, and achieve the effects of safe and effective drug controlled release, uniform diameter distribution, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Implementation of coaxial electrospinning process and preparation of core-sheath nanofibers
[0029] Co-dissolve 1 gram of ibuprofen and 7 grams of polyvinylpyrrolidone K60 in 100 ml of absolute ethanol to prepare the sheath working fluid.
[0030] 1 gram of drug ibuprofen and 9 grams of fiber-forming polymer hydroxypropyl methylcellulose phthalate are co-dissolved in 100ml of absolute ethanol and methylene chloride mixed solvent (volume ratio 1:1), and the preparation into the core working fluid.
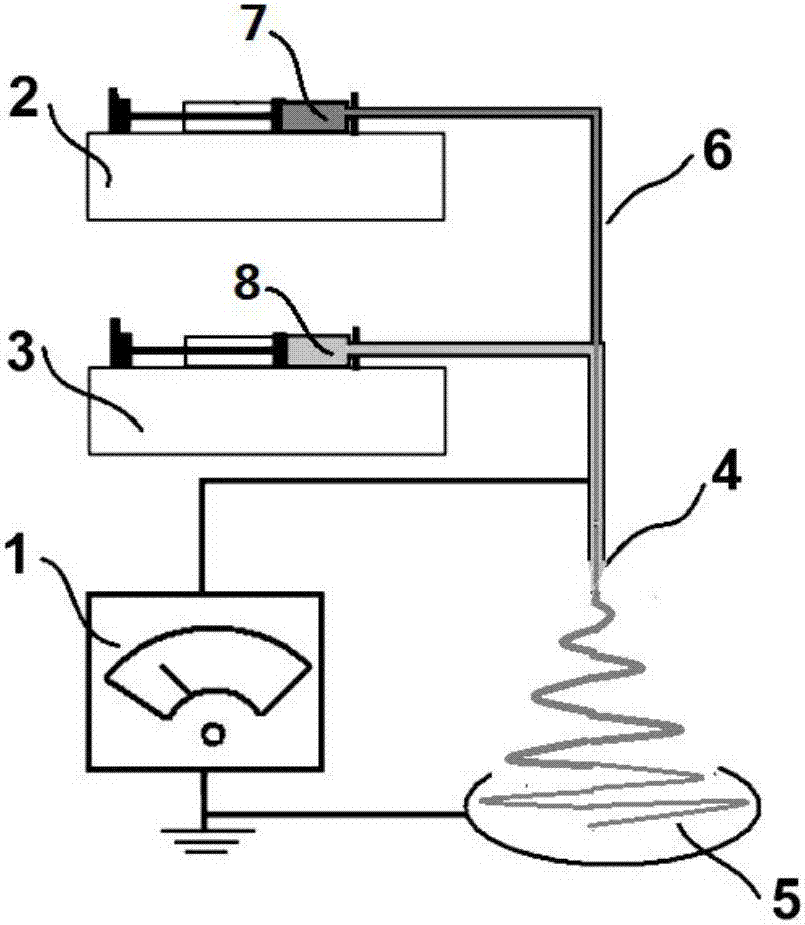
[0031] Put the above-mentioned working fluid into the syringes of the working fluid of the inner core and the outer sheath respectively, install them on the corresponding syringe pumps, and connect them to the two inlets of the coaxial spinning head respectively, connect the high-pressure spinning head and the high-pressure Static generator.
[0032] The injection rate of the core-sheath solution into the coaxial spinneret was controlled by two syringe pumps, th...
Embodiment 2
[0035] Example 2: Structural and Morphological Characterization of Core-Sheath Nanofibers for Secondary Pulse Release of Drugs
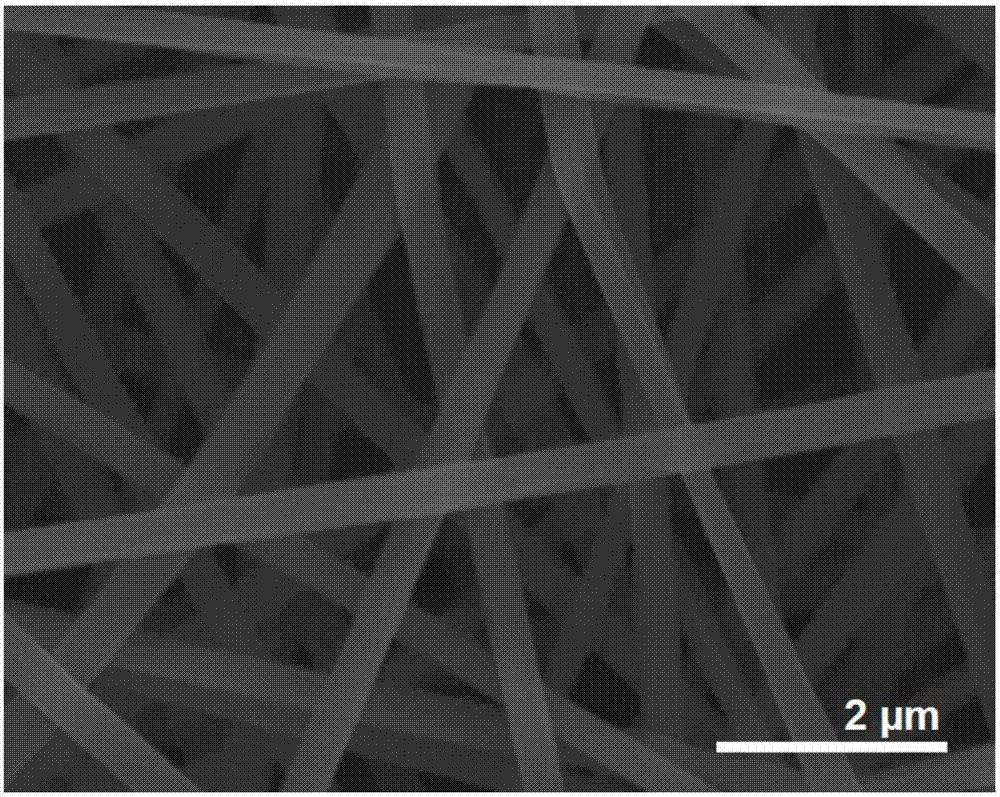
[0036] Field scanning electron microscopy (FESEM) was used to observe the surface of the fiber prepared in Example 1 after spraying gold, and the results were as follows image 3 shown. The prepared fiber exhibits a good linear state, no beading structure occurs, the fiber surface is smooth, and the fiber accumulation is uniform. The diameter is 640±90 nm, the distribution is relatively uniform, and the diameter distribution is relatively concentrated.
[0037] The internal structure of the prepared fiber was observed by high-resolution transmission electron microscopy (TEM), and the results were as follows: Figure 4 As shown, the inner and outer layers of the core-sheath nanofiber have a clear structure, and the internal structure of the fiber is as follows Figure 5 As shown, the drug 33 is uniformly distributed in the outer sheath part 11 of t...
Embodiment 3
[0038] Example 3: Functional Analysis of Drug Controlled Release of Core Sheath Nanofibers with Secondary Pulse Release of Drugs
[0039] According to the 2015 edition of Chinese Pharmacopoeia Appendix ⅩD Release Test Method 2, the RCZ-8A intelligent dissolution tester was used to conduct the in vitro dissolution test on the drug-loaded nanofibers obtained above. The control speed is 50rpm, and the temperature is 37±0.1°C. In the first 2 hours, 900 mL of artificial gastric juice without enzymes was used as the dissolution medium, followed by 900 mL of artificial intestinal juice (pH6.8 phosphate buffer solution) without enzymes as the dissolution medium to investigate the drug release properties of nanofibers in vitro. Sampling 5mL at the scheduled time, filtered through a 0.22 µm microporous membrane to obtain the eluate sample, and immediately replenished with the same volume of isothermal fresh medium. After diluting the sample appropriately, at λ=257 nm, UV-Vis spectropho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com