Polymer nanosphere with function of target recognition of cancer cells and preparation method of polymer nanosphere
A technology for targeting and identifying cancer cells, which can be used in medical preparations, drug combinations, and pharmaceutical formulations that are not active ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
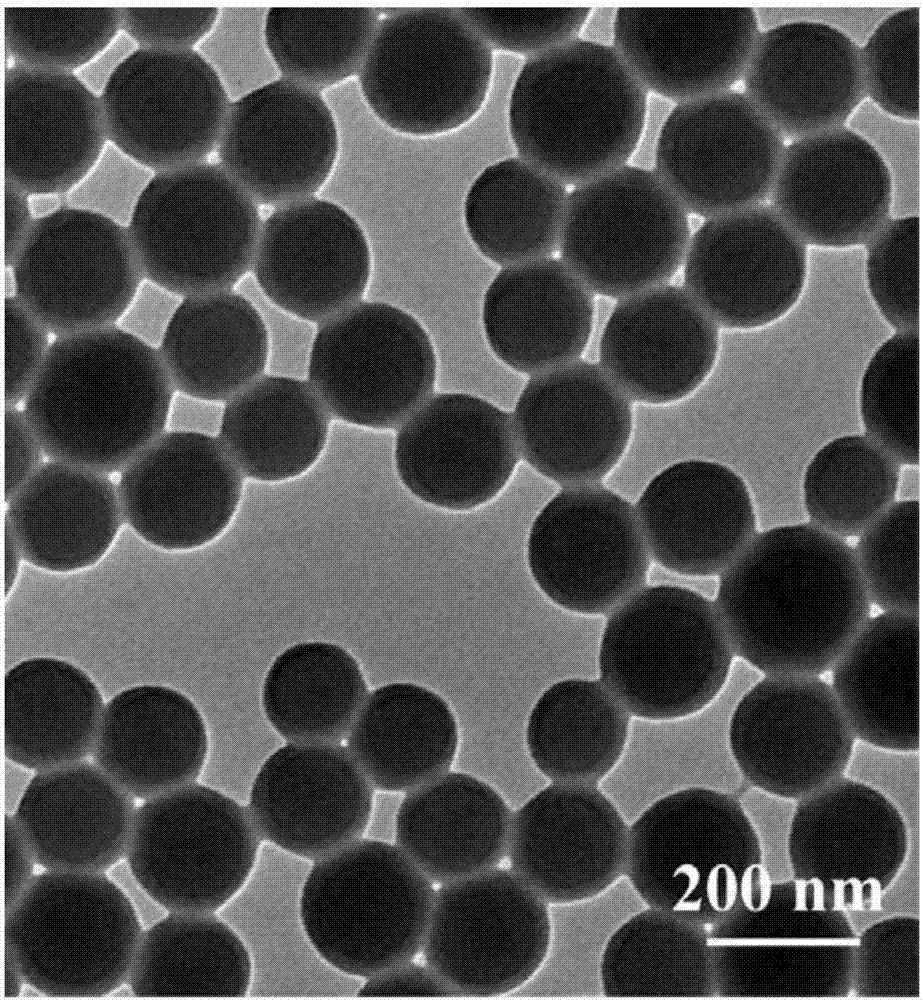
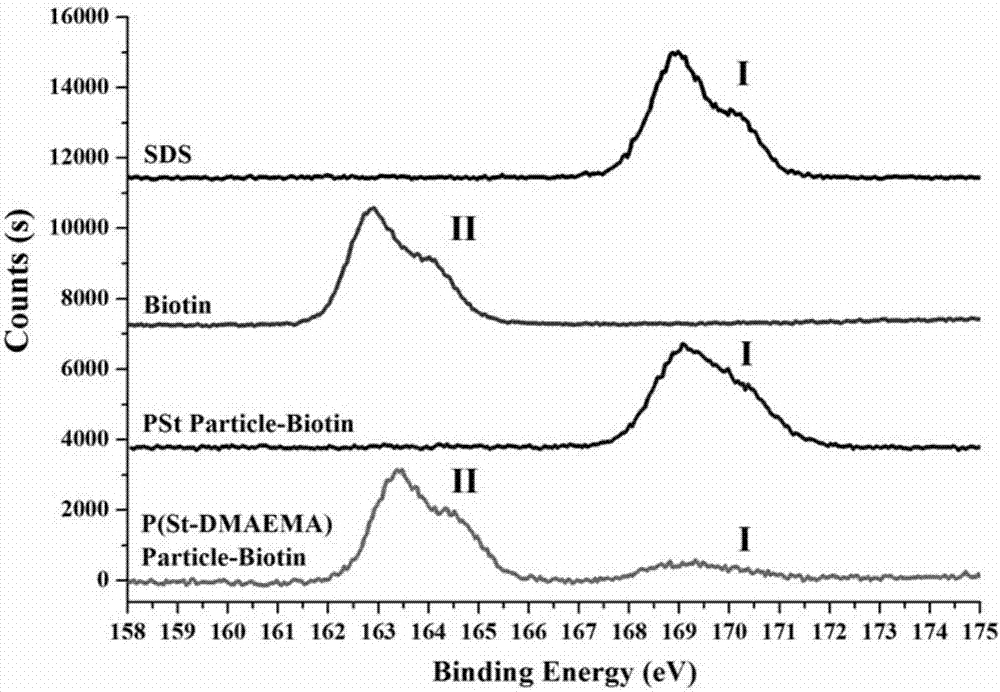
Image
Examples
Embodiment 1
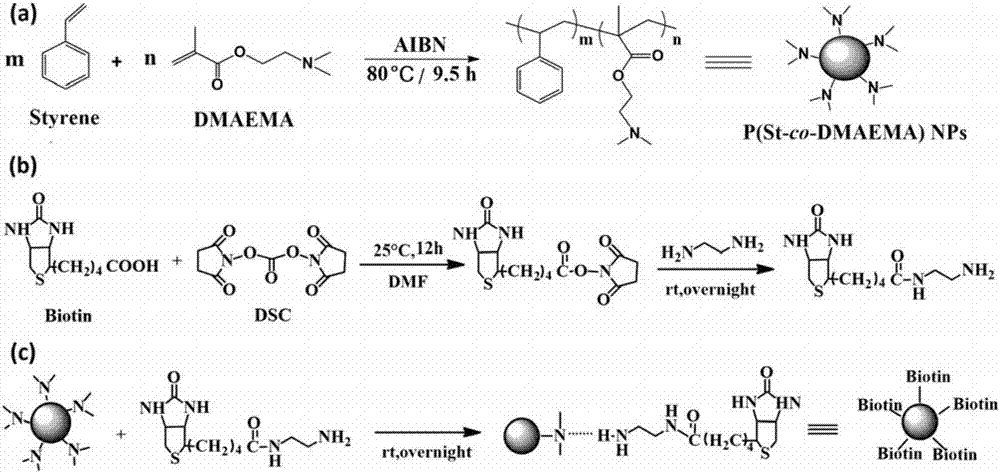
[0059] Embodiment 1, the preparation of the polymer nanosphere of biotin surface modification
[0060] according to figure 1 The schematic diagram for the preparation of biotin-surface-modified polymer nanospheres is shown:
[0061] 1) According to figure 1 Step (a) prepares the polymer nanosphere that surface contains amine group, concrete steps are as follows:
[0062] Dissolve emulsifier 0.2g sodium dodecyl sulfate (SDS) and 0.1g nonylphenol polyoxyethylene ether (OP-10) in water 90mL to obtain emulsifier aqueous solution; initiator azobisisobutyronitrile ( AIBN) is dissolved in a mixed solvent of other copolymerized monomer styrene (styrene), amine-containing monomer dimethylaminoethyl methacrylate (DMAEMA) and cross-linking monomer divinylbenzene (AIBN, styrene The mass ratio of DMAEMA and divinylbenzene is 0.2:6.7:3.3:0.03) to obtain a monomer solution. Pour the emulsifier aqueous solution into a three-neck flask equipped with a spherical condenser and a stirring pad...
Embodiment 2
[0068] Embodiment 2, the preparation of the polymer nanosphere of biotin surface modification
[0069] 1) Preparation of polymer nanospheres containing amine groups on the surface:
[0070] Dissolve 0.2g of cetyltrimethylammonium bromide, 0.1g of nonylphenol polyoxyethylene ether (OP-10) and 0.2g of initiator azobisisobutylamidine hydrochloride in 90mL of water to obtain Aqueous solution containing an emulsifier; other copolymerizable monomers butyl acrylate, methyl methacrylate, amine-containing monomer diethylaminoethyl methacrylate and cross-linking monomer N,N-methylenebis Acrylamide was mixed in a mass ratio of 4:2.5:3.5:0.03 to obtain a monomer solution. Pour the aqueous solution containing the emulsifier into a three-neck flask equipped with a spherical condenser and a stirring paddle, and then drop the monomer solution into the water phase (the mass ratio of the emulsifier aqueous solution to the monomer solution is 9:1). The mixed solution was pre-emulsified using h...
Embodiment 3
[0075] The preparation of the polymer nanosphere of embodiment 3, folic acid surface modification
[0076] 1) Preparation of polymer nanospheres containing amine groups on the surface: the same as in Example 1.
[0077] 2) Synthesis of aminated folic acid: Dissolve folic acid in the organic solvent DMF (10 mg folic acid dissolved in 1 mL DMF), and add N,N'-disuccinimide carbonate equivalent to four times the molar amount of folic acid in an ice-water bath (DSC), under the catalysis of triethylamine (the molar ratio of triethylamine and folic acid is 2:1), react at room temperature (25°C) for 12 hours. The product precipitate was washed three times with diethyl ether, isopropanol and diethyl ether, respectively. Re-dissolve the obtained product in DMF (dissolve 10 mg of product in 1 mL of DMF), then back-drop the solution into excess ethylenediamine (equivalent to 2 times the molar amount of folic acid), and react at room temperature (25°C) for 24 hours . Precipitate and was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com