Preparation method and application of polyoxometalate crystal
A technology of heteropoly acid and crystal, which is applied in the field of preparation of heteropoly acid crystal, can solve the problems of difficult recycling and high pressure of environmental protection treatment, achieve high conversion rate and selectivity, reduce environmental pressure, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
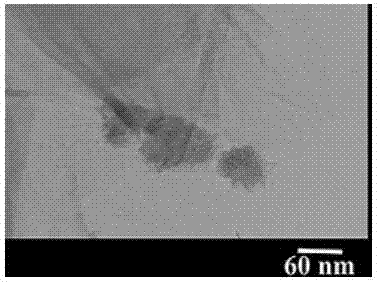
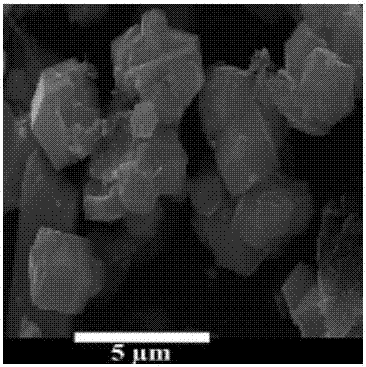
[0034] 1. Preparation of heteropolyacid crystals
[0035] 10g Na 2 WO 4 .2H 2 O and NaVO 3 .2H 2 O (with Na 2 WO 4 .2H 2 O equimolar amount) was dissolved in 100ml water and stirred to dissolve, and then disodium hydrogen phosphate (Na 2 WO 4 .2H 2 3.5 times the molar amount of O) and manganese acetate (Na 2 WO 4 .2H 2 (1.2 times the molar weight) of the aqueous solution was stirred, and after the dropwise addition, the pH was adjusted to 2.2-3.0 with sulfuric acid; 2 WO 4 .2H 2 O 4.2 times the molar amount) aqueous solution was stirred and reacted for 1-2h to obtain a turbid solution; adding potassium acetate to adjust the pH to 4.1-5.2, and a large amount of black solid was precipitated; filtering and washing the filter cake with purified water and ethanol in turn, at 40-45°C Drying; the solid obtained after drying is recrystallized with isopropyl acetate to obtain heteropolyacid crystals, and the heteropolyacid crystals are characterized by transmission elect...
Embodiment 2
[0040] With reference to the reaction process of Example 1, using the above-mentioned prepared heteropolyacid crystal as a catalyst, and using 10g (I) formula as a raw material, the reaction solvent (all of which is 7 times the weight of the substrate), reaction temperature, and heteropolyacid crystal consumption were investigated. (calculated based on the raw material (I) formula structure) on the conversion rate of (I) formula compound and the product (II) formula yield and its purity, the results are shown in Table 1:
[0041] The catalytic effect of table 1 different reaction conditions
[0042]
[0043] Note: Yield refers to the percentage of (isolation weight X purity) / theoretical yield; purity refers to the HPLC purity of the separated product, area percentage.
[0044] The above results show that the conversion rate of raw materials in ester solvents is high; at a reaction temperature of 10-15 ° C, the selectivity of raw materials is high, and the purity of products...
Embodiment 3
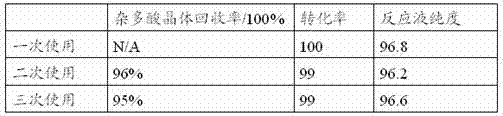
[0046] Using ethyl acetate as the solvent, the reaction temperature is 10-15°C, and the catalyst dosage is 2%wt. Referring to the preparation process of the compound of formula (II) in Example 1, the influence of the mechanical application of the catalyst on the reaction is investigated. The results are shown in Table 2:
[0047] Table 2 The influence of the application times of heteropolyacid crystals on the reaction
[0048]
[0049] Note: The purity of the reaction solution refers to the purity of the reaction solution after the detected reaction, not the purity of the crude product obtained after post-processing; the recovery rate of heteropoly acid crystals refers to the weight of the heteropoly acid crystals obtained by filtering and drying after the reaction and the weight of the feed before the reaction percentage.
[0050] The above results show that the conversion rate of the raw material and the selectivity of the product do not decrease significantly after the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com