A kind of streptococcus suis truncated protein sao and its application
A technology of Streptococcus suis and truncated protein, applied in application, antibacterial drugs, veterinary vaccines, etc., can solve the problems of difficult purification of full-length sao protein, hindering the research and development of Sao protein vaccine, poor protein stability, etc. It is not easy to achieve Degradation, stable properties and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthesis and vaccine preparation of the truncated protein Sao of Streptococcus suis:
[0030] 1.1 Synthesis of truncated protein Sao
[0031] The sequence of the truncated protein Sao is shown in SEQ ID NO. 2, synthesized by Shenggong Bioengineering (Shanghai) Co., Ltd. After synthesis, the truncated protein Sao polypeptide has a purity of 99%, 5mg each, in white powder form, stored at -20 degrees in the dark, and is completely soluble in water. The protein was dissolved in a PBS buffer solution, stored at -80°C for one month and then taken out and no degradation of the protein was found.
[0032] Before use, use sterile PBS (NaCl 8.0g, KCl 0.2g, KH 2 PO 4 0.24g, Na 2 HPO 4 ×12H 2 O 3.628g, pH7.4) Dilute according to the specified concentration. Preheat in a 37 degree incubator before use.
[0033] 1.2 Preparation of vaccine
[0034] Dosage form: water-in-oil type (W / O)
[0035] Adjuvant composition: white mineral oil (Marcol52), Tween 80 (CRILLET4), Span 80 (CRILL4).
[0...
Embodiment 2
[0046] Application of truncated protein vaccine Sao in preparation of vaccine against Streptococcus suis infection
[0047] 1. Evaluate the immune protection effect of the vaccine on a mouse model
[0048] 1.1 Experimental protocol
[0049] Experimental animals: 4-week-old female BALB / c mice, purchased from Hubei Provincial Center for Disease Control and Prevention.
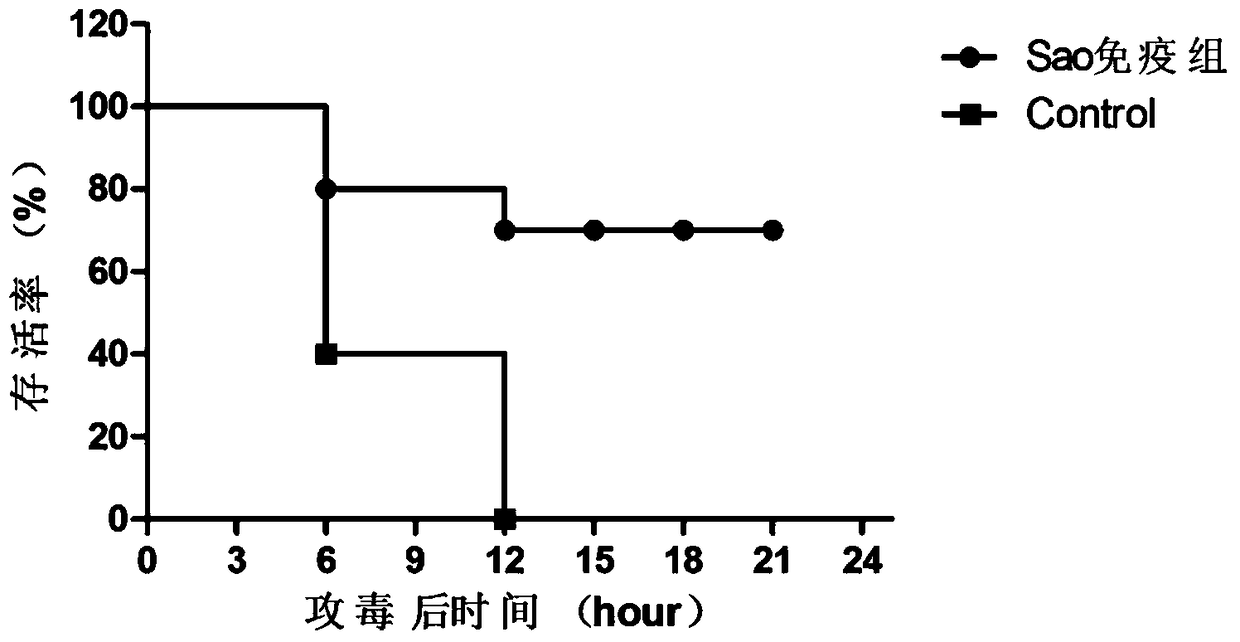
[0050] Challenge strain: Streptococcus suis type 2 SC19.
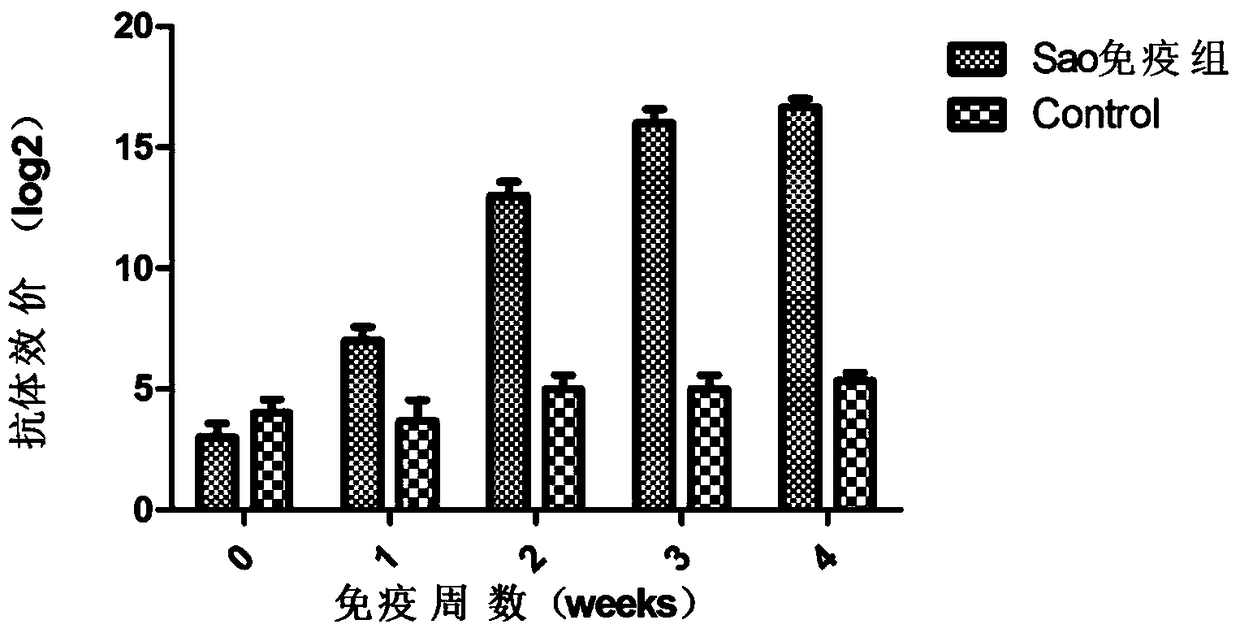
[0051] Experimental grouping: 20 female BALB / c mice weighing 16g were randomly divided into 2 groups: vaccine group I (Sao), control group (adjuvant and PBS), 10 mice in each group. During the post-immunization period, blood was collected from the tail every week for monitoring of serum specific antibodies. Immunization method: The vaccine prepared in Example 1 was subcutaneously immunized with a dose of 100 μl / mouse; a booster immunization was performed 2 weeks later (the dose and location of the immunization were the same as the first immunization).
[0052] 1.2...
Embodiment 3
[0075] Example 3: Safety inspection
[0076] In order to examine the safety effects of the prepared vaccine on piglets and pregnant sows, safety inspections were carried out on 35-day-old piglets and 70-day-old sows.
[0077] 3.1 Experimental program:
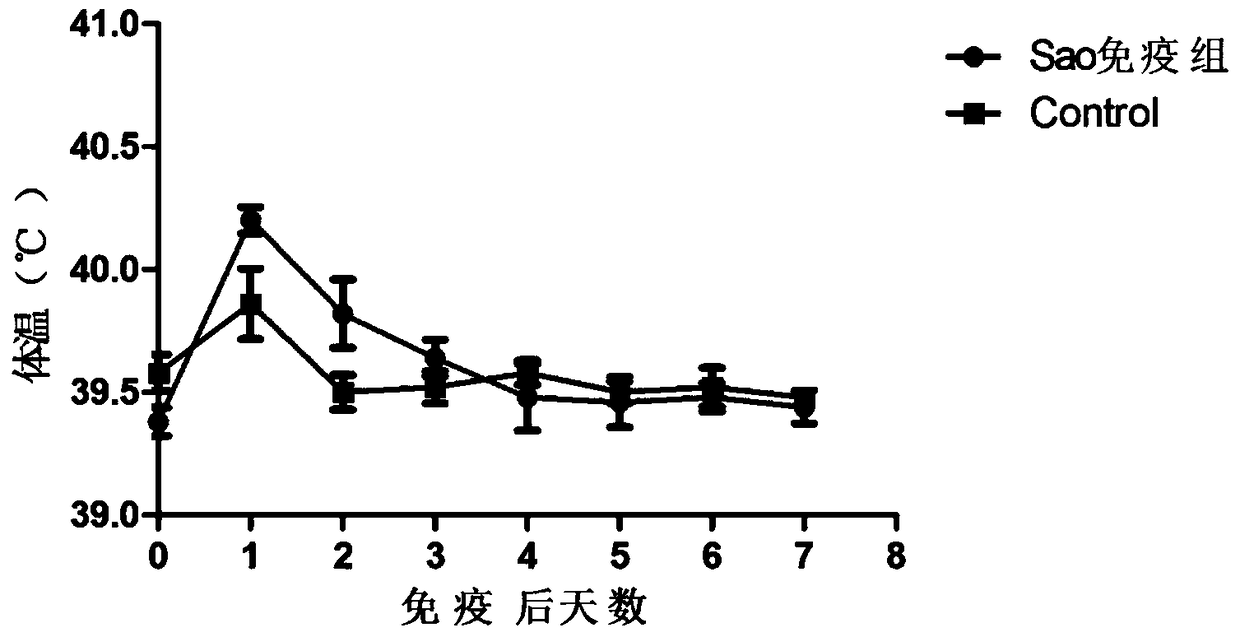
[0078] 1) Inoculate 35-day-old healthy piglets through separate injections of the prepared vaccines into the hip muscles. Each batch of vaccines will be injected with 5 piglets, each with 2ml, and observe the clinical manifestations and measure the body temperature.
[0079] 2) The prepared vaccines were injected into healthy sows on the 70th day of gestation through separate injections into the hip muscles. Each batch of vaccines were injected with 5, 2ml each, and the clinical manifestations and farrowing conditions were observed.
[0080] 2. Test results
[0081] 3.2.1 The safety of the subunit vaccine of the present invention for vaccination of weaned piglets
[0082] After the prepared vaccine was inoculated with 35-day-old healthy we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com