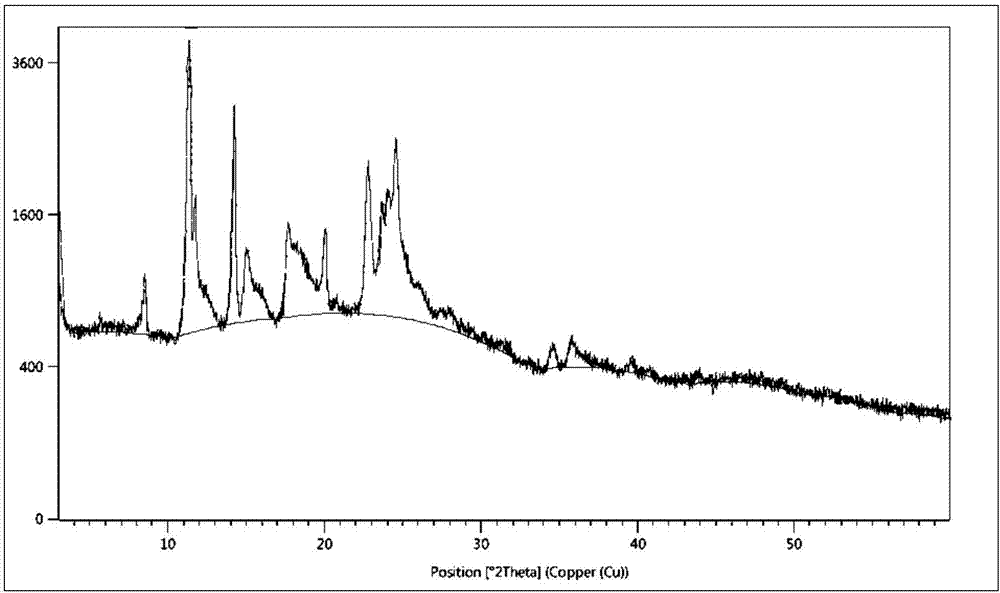
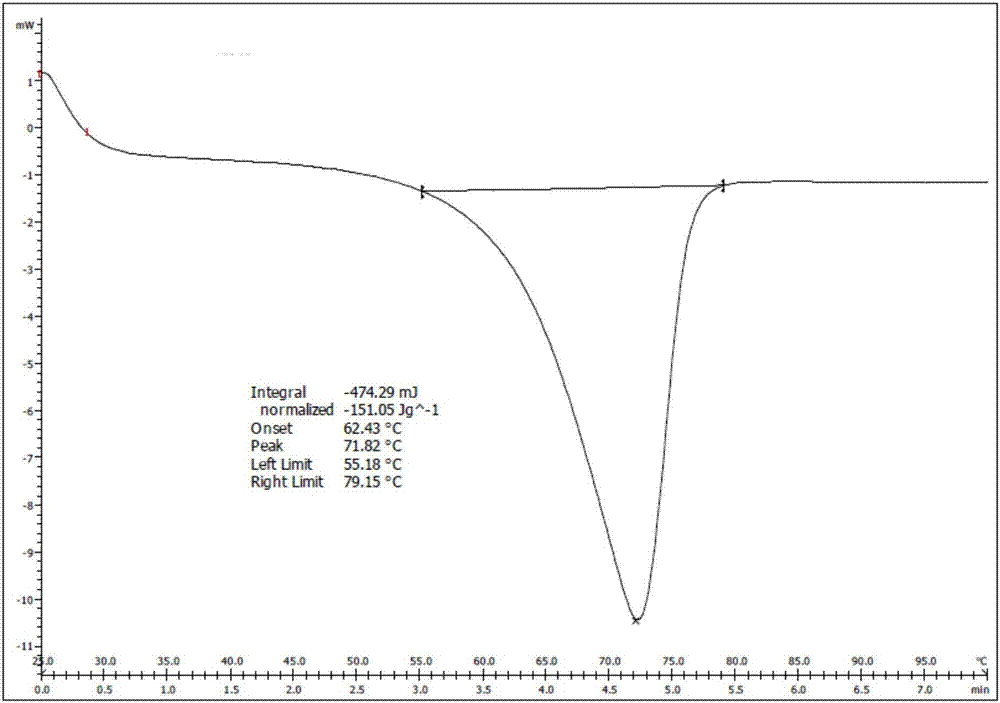
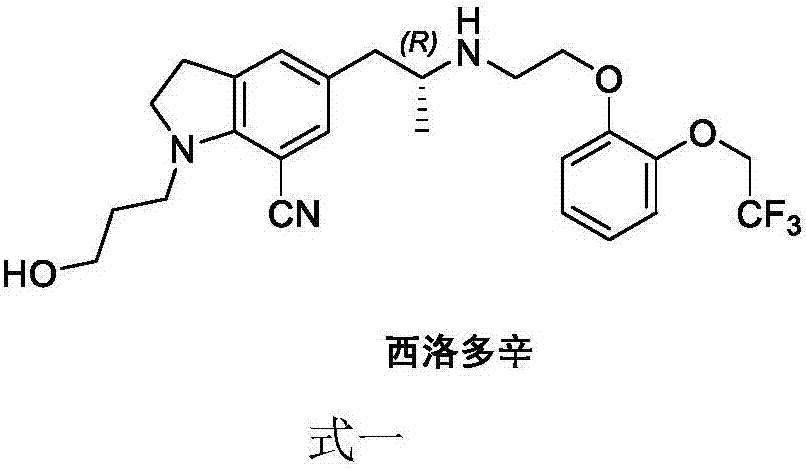
Synthesizing method of silodosin and intermediate thereof
A synthesis method and technology for silodosin, which are applied in the field of synthesis of silodosin and its intermediates, can solve the problems of many protection and deprotection steps, unsuitable control of process conditions, lengthy route steps and the like, and reduce industrial production costs. and risk, improve yield, the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 compound 3
[0042]
[0043] At room temperature, drop 251g compound 2, 1500mL 30% hydrochloric acid, 1000mL acetic acid into the reaction vessel, optionally, the feed ratio (mass) of compound 2, hydrochloric acid and acetic acid is 1:5~10:5~10; then Slowly raise the temperature to 80-90°C for reaction, monitor by liquid chromatography until the reaction is complete (4-10 hours), concentrate the reaction solution to dryness under reduced pressure, add 1500mL dichloromethane and 2000mL cold saturated sodium carbonate aqueous solution, and stir for 1 hour The layers were separated, the organic layer was washed with saturated brine, the collected organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 203 g of compound 3 as a yellow solid, yield: 97%. Melting point: 91-93°C; HRMS m / z (ESI): C 11 h 13 C1NO[M+H + ]Theoretical calculation value: 210.068...
Embodiment 2
[0044] Embodiment 2: the preparation of compound 4
[0045]
[0046] At room temperature, in an organic solvent, put 209g of compound 3, 194g of potassium phthalimide, 1000mL of acetonitrile, and 151g of potassium carbonate into the reaction vessel. Optionally, the organic solvent can be selected from acetonitrile, toluene, tetrahydrofuran Among others, acetonitrile is preferred. Wherein the molar ratio of compound 3, potassium phthalimide and potassium carbonate can be 1:1.0-2.0:1.0-2.0; preferably 1:1.1:1.05.
[0047] Then slowly warming up to 80-90 ℃ of reaction, liquid chromatographic monitoring until the reaction is complete (4-10 hours), the reaction solution was concentrated to dryness under reduced pressure, added in 1500mL dichloromethane and 2000mL cold saturated sodium carbonate aqueous solution, stirred for 1 After hours, the layers were separated, the organic layer was washed with saturated brine, the collected organic layer was dried over anhydrous sodium sul...
Embodiment 3
[0048] Embodiment 3: the preparation of compound 5
[0049]
[0050] At room temperature, in an organic solvent, put 48g of compound 4, 32g of 3-chloropropyl benzoate, 26g of potassium iodide, 33g of potassium carbonate, 2.2g of tetrabutylammonium bromide, 600mL of DMF into the reaction flask, optional Yes, the organic solvent is selected from dimethylformamide, dimethylacetamide, dimethyl sulfoxide, nitrogen methylpyrrolidone, preferably dimethylformamide. Wherein the molar ratio of compound 4 to 3-chloropropyl benzoate, potassium carbonate, potassium iodide, and phase transfer catalyst tetrabutylammonium bromide can be selected as 1: 1.0~2.0: 1.0~2.0: 2.0~4.0: 0.05~0.1 , preferably 1: 1.6: 2.4: 1.6: 0.07; wherein the phase transfer catalyst can also be selected from tetrabutylammonium chloride, tetrabutylammonium iodide, benzyl trimethyl ammonium chloride or benzyl triethyl chloride ammonium chloride.
[0051] Then slowly raise the temperature to 90-100°C for reaction, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com