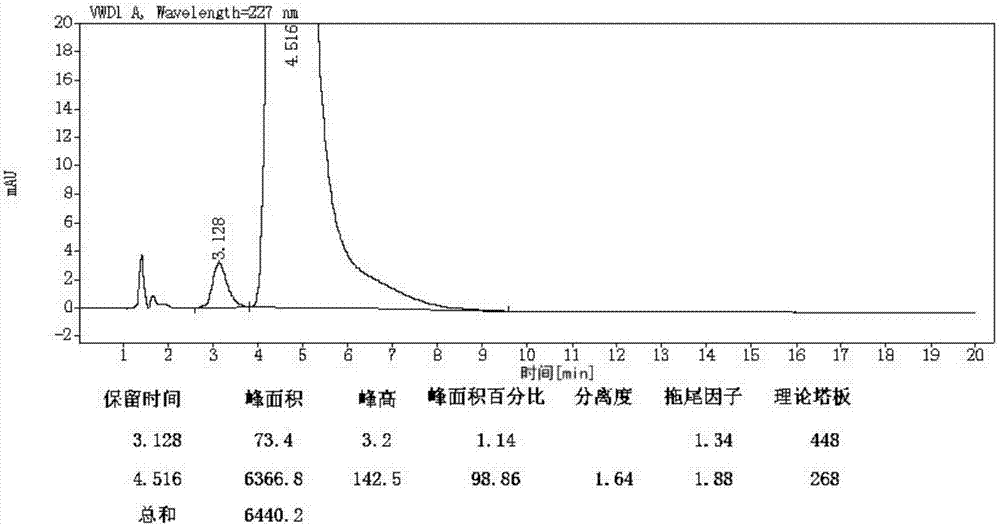
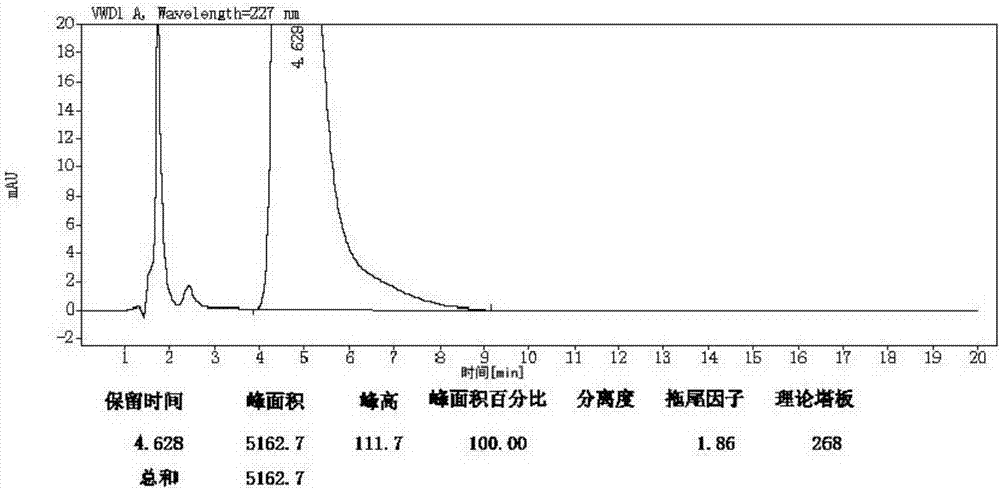
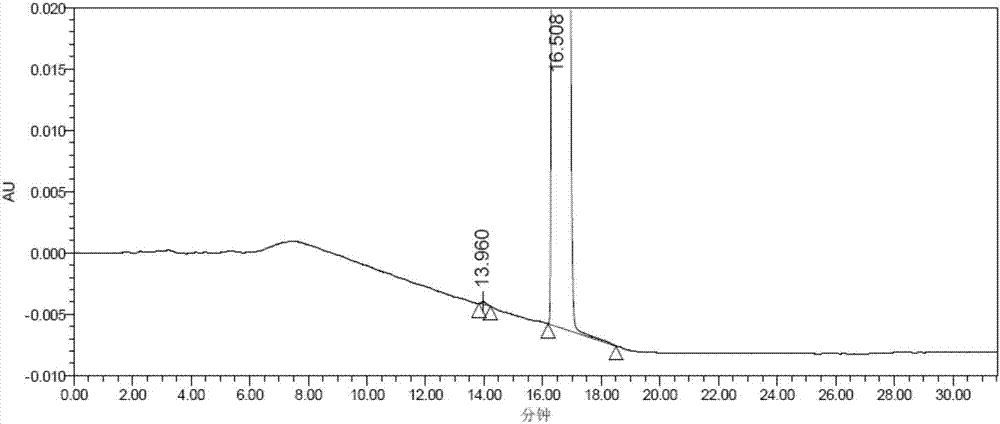
Preparation and purification method of valsartan
A technology for valsartan and valine, which is applied in the field of preparation and purification of valsartan, can solve the problems of difficult control of tin content, difficult operation, long reaction time, etc., and achieves the avoidance of tin metal residue, stable process, The effect of process optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A preparation method of valsartan, comprising the following steps:
[0060] Step S1. Add 5kg (that is, 42.7mol) of L-valine and 4kg of absolute ethanol into a 50L glass reactor, stir to dissolve, cool down to 0-10°C, slowly add 8.8L of thionyl chloride dropwise, drop After the addition, heat up and reflux for 18 hours, concentrate to dryness, add 15L of isopropyl acetate at 15-20°C for beating for 1 hour, and filter with suction to obtain 6.1 kg of off-white solid intermediate 1, wherein R 1 For ethyl. The spectral information of intermediate 1 is: 1H-NMR (400MHz, CDCl3, δ: 0.91[t, 3H, -CH3]), 0.93[t, 3H, -CH3]), 1.29[t, 3H, -CH3] ), 2.67 [m, 2H, -CH2]), 4.21 [m, 2H, -CH2]), 4.25 [t, 1H, -CH]).
[0061]
[0062] Step S2. Add 6kg of intermediate 1 and 72mol of starting material 2 (2-cyano-4'-bromomethylbiphenyl) and 60kg of ethyl acetate into a 150L glass reactor, stir evenly, and add 48kg of sodium carbonate aqueous solution (containing 144mol of sodium carbonate)...
example 2
[0071] A preparation method of intermediate 1:
[0072] Step S1. Add 5kg (that is, 42.7mol) of L-valine and 4kg of anhydrous methanol into a 50L glass reactor, stir to dissolve, cool down to 0-10°C, slowly add 6.6L of thionyl chloride dropwise, dropwise After completion, heat up and reflux for 15 hours, concentrate to dryness, add 15L isopropyl acetate at 15-20°C for 2 hours, and filter with suction to obtain 5.5 kg of off-white solid intermediate 1, R 1 For methyl.
[0073]
example 3
[0075] A preparation method of intermediate 1:
[0076] Add 5kg (that is, 42.7mol) of L-valine and 4kg of anhydrous butanol into a 50L glass reactor, stir to dissolve, cool down to 0-10°C, slowly add 15.2L of thionyl chloride dropwise, and the dropwise addition is completed Afterwards, heat up and reflux for 16 hours, concentrate to dryness, add 15L of isopropyl acetate at 15-20°C for beating for 1 hour, and filter with suction to obtain 6.2 kg of off-white solid intermediate 1, R 1 For butyl:
[0077]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com