Er<3+>-doped vanadium germanate light conversion material and preparation method thereof
A light conversion material, germanate technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of weak absorption and low resonance efficiency, and achieve the effects of no impurity phase, pure phase, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
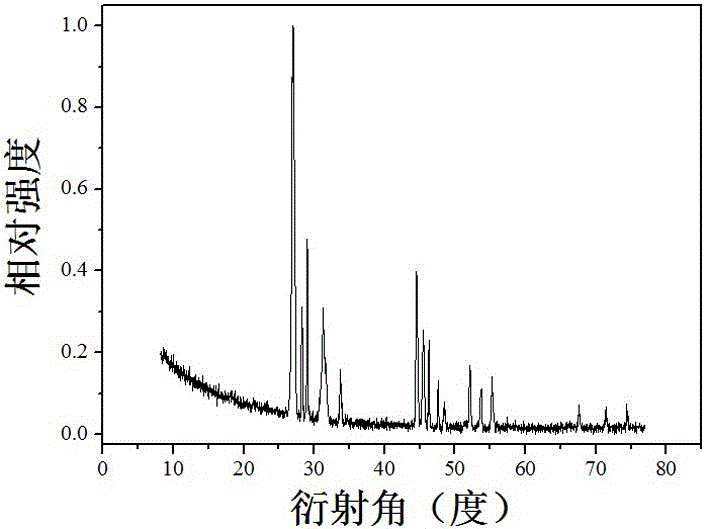
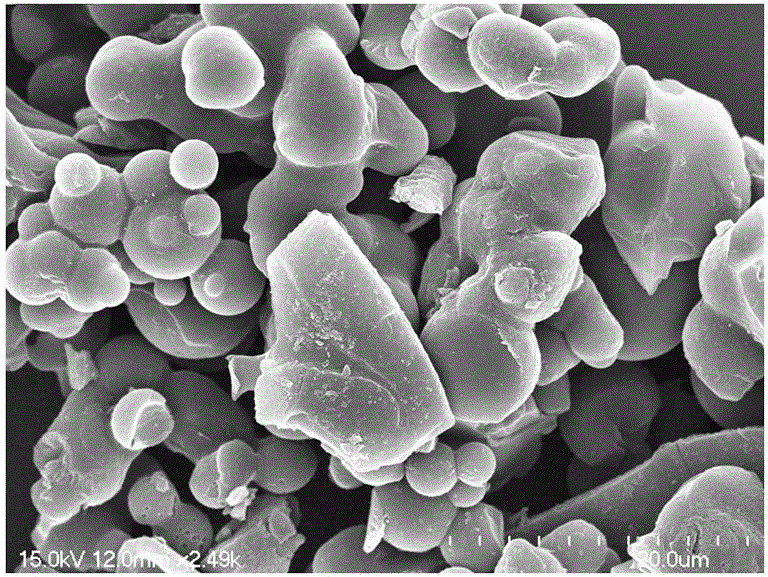
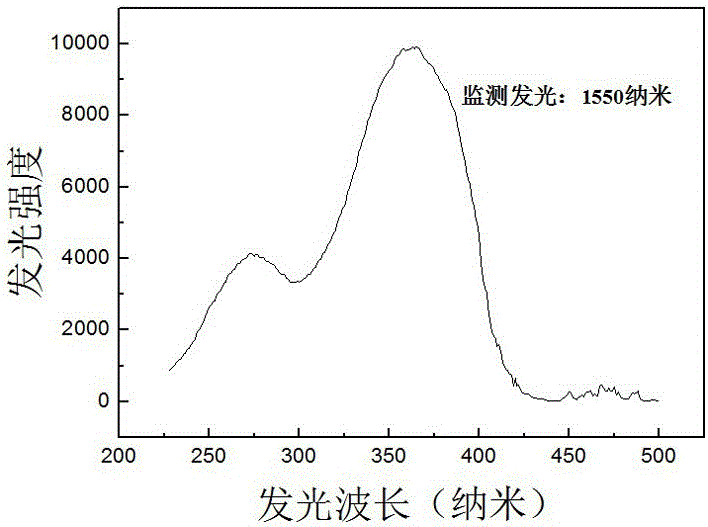
Image
Examples
Embodiment 1
[0037] According to the chemical formula La 10.9989 Er 0.0011 GeV 3 O 26 The stoichiometric ratio of each element in the lanthanum nitrate La(NO 3 ) 3 ·6H 2 O: 7.9375 grams, Erbium Nitrate (NO 3 ) 3 ·5H 2 O: 0.0008 g, germanium chloride GeCl 4 :0.3573 grams, ammonium metavanadate NH 4 VO 3 : 0.5849 grams. Dissolve lanthanum nitrate and erbium nitrate in an appropriate amount of dilute nitric acid, add 5.2839 grams of citric acid weighed and stir magnetically at 70°C for a period of time to obtain a clear solution. Dissolve germanium chloride and ammonium metavanadate in a deionized aqueous solution. Add 1.9214 g of citric acid and stir at 70°C to obtain a clear solution.
[0038] The above-prepared solution was mixed and stirred at 50°C for 1.5 hours, and the obtained mixed solution was placed in an oven with a set temperature of 80°C. After drying for 12 hours, it was naturally cooled and the precursor was taken out. The precursor is placed in a muffle furnace, calcined in an ai...
Embodiment 2
[0044] According to the chemical formula La 6.05 Er 4.95 GeV 3 O 26 The stoichiometric ratio of each element in the lanthanum oxide La 2 O 3 :1.6428g, Erbium oxide 2 O 3 :1.5779 grams, germanium chloride GeCl 4 :0.3573 grams, vanadium pentoxide V 2 O 5 : 0.455 grams. Dissolve lanthanum oxide and erbium oxide in an appropriate amount of dilute nitric acid, and add weighed 3.287 g of oxalic acid and stir magnetically at 100°C for a period of time to obtain a clear solution. Dissolve germanium chloride and vanadium pentoxide in a deionized aqueous solution, and add 1.21 g of oxalic acid was stirred at 100°C to obtain a clear solution.
[0045] Finally, the above two solutions were mixed and stirred at 100°C for 1 hour. The obtained mixed solution was dried in an oven, cooled naturally, and the precursor was taken out. The precursor was placed in a muffle furnace and calcined in an air atmosphere. The sintering temperature was 1200℃, calcination time is 10 hours, cooled to room tempe...
Embodiment 3
[0048] According to the chemical formula La 10.45 Er 0.55 GeV 3 O 26 The stoichiometric ratio of each element in the lanthanum oxide La 2 O 3 :2.8375 grams, Erbium nitrate Er(NO 3 ) 3 ·5H 2 O: 0.4064 g, germanium chloride GeCl 4 :0.3573 grams, ammonium metavanadate NH 4 VO 3 :0.5849g; Dissolve lanthanum oxide in an appropriate amount of dilute nitric acid, and add 3.93g of oxalic acid weighed and stir at 80℃ to obtain a clear solution; Dissolve erbium nitrate in deionized water, add 0.5g of oxalic acid and stir at 85℃ Obtain a clear solution; dissolve germanium chloride and ammonium metavanadate in a deionized aqueous solution, add 1.5 g of oxalic acid and stir at 85°C to obtain a clear solution.
[0049] Finally, the above solution was mixed and stirred at 50°C for 5 hours. The obtained mixed solution was dried in an oven, cooled naturally, and the precursor was taken out. The precursor was placed in a muffle furnace and calcined in an air atmosphere. The sintering temperature was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com