ESIPT (excited state intramolecular proton transfer) type fluorescent probe for biological mercaptan detection and application
A fluorescent probe, thiol technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., to achieve the effects of simple synthesis steps, good stability, and strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of ESIPT-type fluorescent probes for biothiol detection
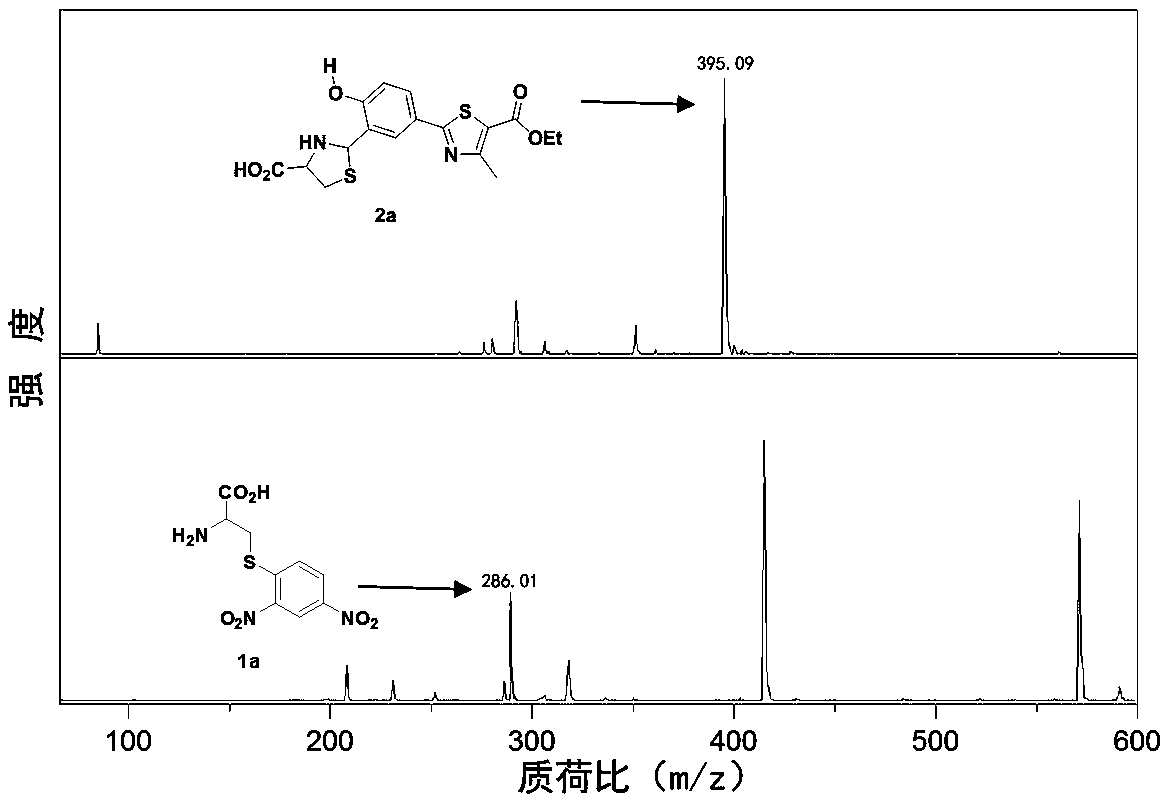
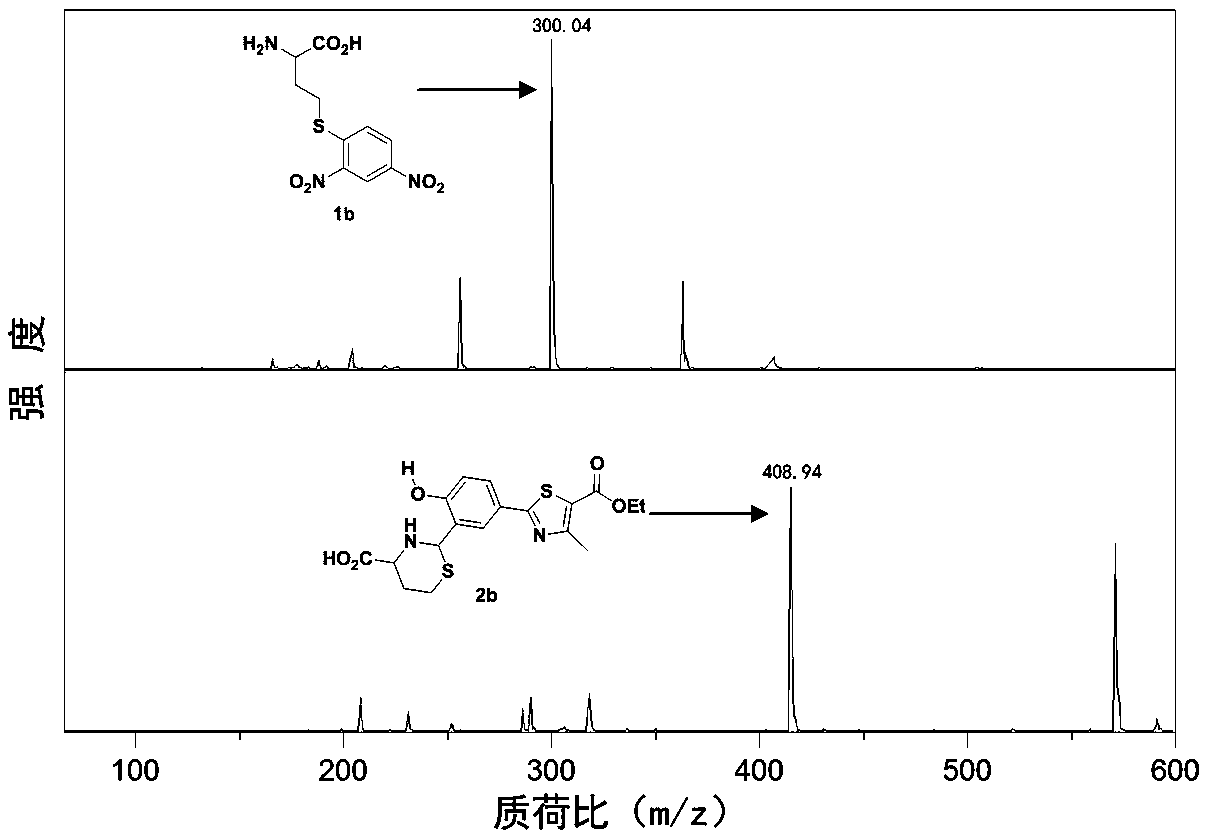
[0045] The ESIPT fluorescent probe is ethyl 2-(4-(2,4-dinitrophenylsulfonyloxy)-3-formylphenyl)-4-methylthiazole-5-carboxylate (NL- S). 2-(4-(2,4- Dinitrophenylsulfonyloxy)-3-formylphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester, the generated 2-(4-(2,4-dinitrophenylsulfonyl Acyloxy)-3-formylphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester (NL) can react with biothiol to remove 2,4-dinitrophenylsulfonyloxy Releases the fluorescent ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-2-carboxylate, followed by 2-(3-formyl-4-hydroxyphenyl)-4- Ethyl methylthiazole-2-carboxylate continues to react with cysteine (Cys) and homocysteine (Hcy) to form a Schiff base, respectively to obtain 2-(5-(5-(B Oxycarbonyl)thiazol-2-yl)-2-hydroxyphenyl)-1,3-thiadioxane-4-carboxylic acid and 2-(5-(5-(ethoxycarbonyl)thiazol-2-yl )-2-hydroxyphenyl)thiazolidine-4-carboxylic acid, so as to achieve the det...
Embodiment 2
[0056] For the detection of biothiols in solution systems
[0057] For the detection of biothiols in 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) / dimethylsulfoxide (DMSO) buffer, use 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) / dimethyl Fluorescent probe working solution with a concentration of 10 μM was prepared with sulfoxide (DMSO) buffer and ESIPT-type fluorescent probe; the specific operation process was as follows:
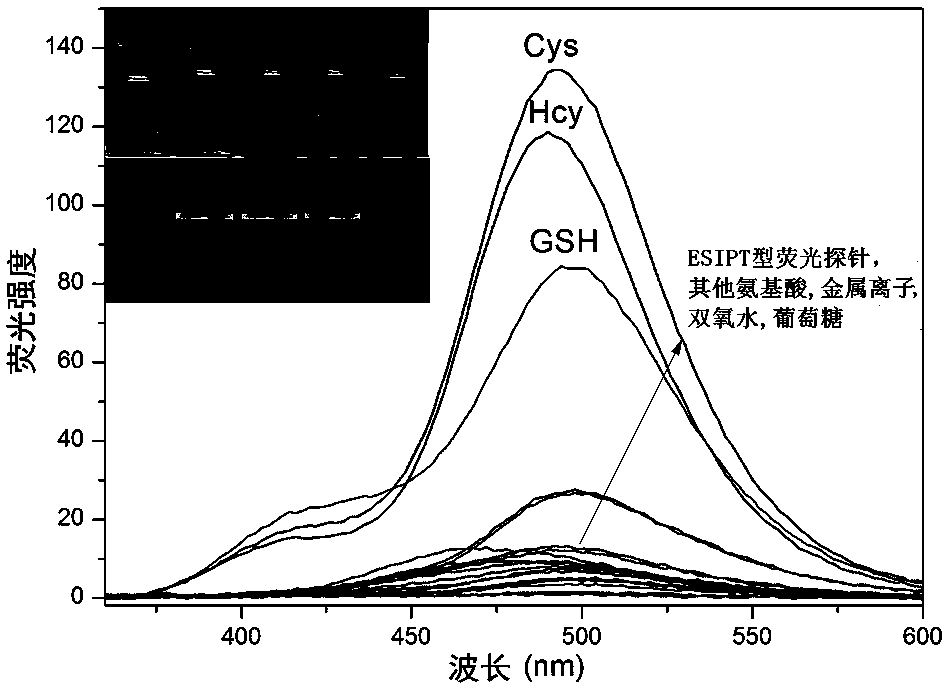
[0058] Use a pipette gun to add 2-(4-(2,4-dinitrophenylsulfonyloxy)-3-formylphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester to 3 mL In the solution of HEPES / DMSO buffer (pH=7.4, v / v=2:8, 10μM), add 200μM cysteine (Cys), homocysteine (Hcy), glutathione Peptide (GSH), proline, aspartic acid, tryptophan, arginine, tyrosine, histidine, gluten glutamic acid, lysine, threonine, glycine, potassium nitrate (KNO 3 ), calcium nitrate (Ca(NO 3 ) 2 ), sodium nitrate (NaNO 3 ), magnesium nitrate (Mg(NO 3 ) 2 ), copper nitrate (Cu(NO 3 ) ...
Embodiment 3
[0060] Intracellular fluorescence imaging detection
[0061] For the detection of biothiols in human tumor cells HeLa cells, use 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) / dimethyl sulfoxide (DMSO) buffer and ESIPT fluorescent probe to prepare a concentration of 20 μM Fluorescent probe working solution; the specific operation process is as follows:
[0062]Take A, B, C, D four groups, A group: HeLa cells; B group: at 37 ℃, HeLa cells with 20μM probe 2-(4-(2,4-dinitrophenylsulfonyloxy )-3-formylphenyl)-4-methylthiazole-5-carboxylate ethyl ester treatment for 0.5 hours; Panel C: HeLa cells with 1mM N-ethylmaleimide (NEM) in cell culture medium Incubate for 2 hours, wash three times with PBS buffer, and then wash with 20 μM 2-(4-(2,4-dinitrophenylsulfonyloxy)-3-formylphenyl)-4-methylthiazole- Ethyl 5-carboxylate solution was incubated for 0.5 hours; in group D, HeLa cells were incubated with 1 mM NEM in cell culture medium for 2 hours, washed three times with PBS buffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com