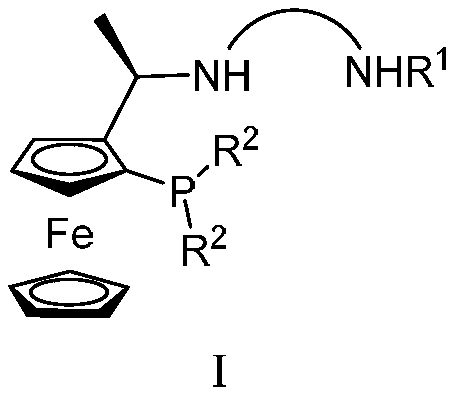
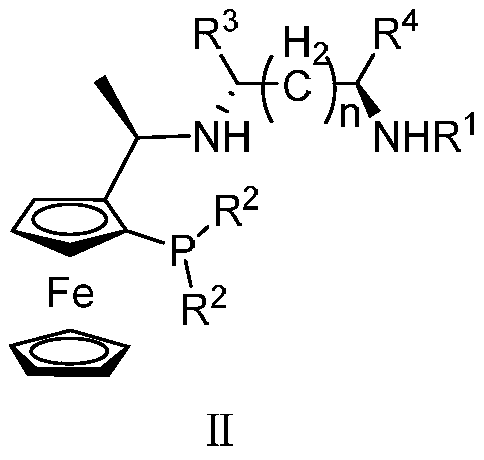
Tridentate Nitrophosphine Ligands and Their Complexes, and Their Applications in Asymmetric Catalytic Hydrogenation of Ketones
A technology of tridentate nitrogen and phosphine ligands, applied in organic compound/hydride/coordination complex catalysts, catalytic reactions, preparation of organic compounds, etc., can solve the problems of chiral alcohols that need to be improved, and achieve convenient storage and Use, high activity, high conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of tridentate nitrogen phosphine ligand
[0043]
[0044] 0°C, N 2 Add 7mL of tBuLi's n-hexane solution (1.6mol / L, 11.2mmol) dropwise into anhydrous ether (20mL) solution of compound 1 (2.57g, 10mmol) under stirring, and naturally rise to room temperature and stir 2h. Then the temperature was lowered to -78°C, and redistilled PCl was slowly added dropwise. 3 (11.46 mmol, 1 mL), the mixture was warmed to room temperature and reacted overnight. Then the temperature was lowered to -78°C again, and R was slowly added dropwise with a constant pressure funnel. 2 MgBr solution (by 30mmol R 2 Br and 0.8g, 33.3mmol magnesium chips were prepared in tetrahydrofuran). After the dropwise addition, the temperature was slowly raised to react overnight, and then 20 mL of saturated NH 4 Cl solution. The oily phase was extracted three times with ether, each time with 20 mL of ether. The oil phase was dried with anhydrous sodium sulfate, spin-dried, and chromatographe...
Embodiment 2
[0060] Preparation of 1-phenylethyl alcohol from acetophenone (S / C=10 000)
[0061]
[0062] Under high-purity argon atmosphere, [Ir(COD)Cl] 2 (3.4mg, 0.005mmol) and chiral ligand L6 (9.2mg, 0.011mmol) were dissolved in isopropanol (1mL) and stirred at room temperature for 3 hours to obtain a clear orange solution. Take 20 μL (0.001 mol%) of the orange solution with a microsyringe and add it to a mixed system of acetophenone (2 mmol), isopropanol (2 mL) and lithium tert-butoxide (1 mol %). The reaction system was placed in an autoclave at room temperature and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate) to obtain pure product 1-phenylethanol. The product was analyzed by HPLC and the measured ee value was 98%. Determination of enantiomeric excess by HPLC, Chiralcel OD-H column, n-hexane:isopropanol=95:5; flow rate=1.0mL / min; UV detection at 210nm...
Embodiment 3
[0064] Preparation of 1-phenylpropanol from propiophenone (S / C=10 000)
[0065]
[0066] Under high-purity argon atmosphere, [Ir(COD)Cl] 2 (3.4mg, 0.005mmol) and chiral ligand L6 (9.2mg, 0.011mmol) were dissolved in isopropanol (1mL) and stirred at room temperature for 3 hours to obtain a clear orange solution. Take 20 μL (0.001 mol%) of the orange solution with a microsyringe and add it to a mixed system of propiophenone (2 mmol), isopropanol (2 mL) and lithium tert-butoxide (1 mol %). The reaction system was placed in an autoclave at room temperature and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate) to obtain pure 1-phenylpropanol. The product was analyzed by HPLC, and the measured ee value was 99%. Determination of enantiomeric excess by HPLC, Chiralcel OJ-H column, n-hexane:isopropanol=95:5; flow rate=1.0mL / min; UV detection at 210nm; t R (S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com