BSA (Bovine Serum Albumin)-gadolinium ion complex wrapped hollow golden nano shell and preparation method
An ionic complex and gold nanoshell technology, which is applied in the field of hollow gold nanoshells and preparation, can solve problems such as no hollow gold nanoshells reported yet, and achieves stable and reliable preparation methods, readily available raw materials, and strong reaction controllability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
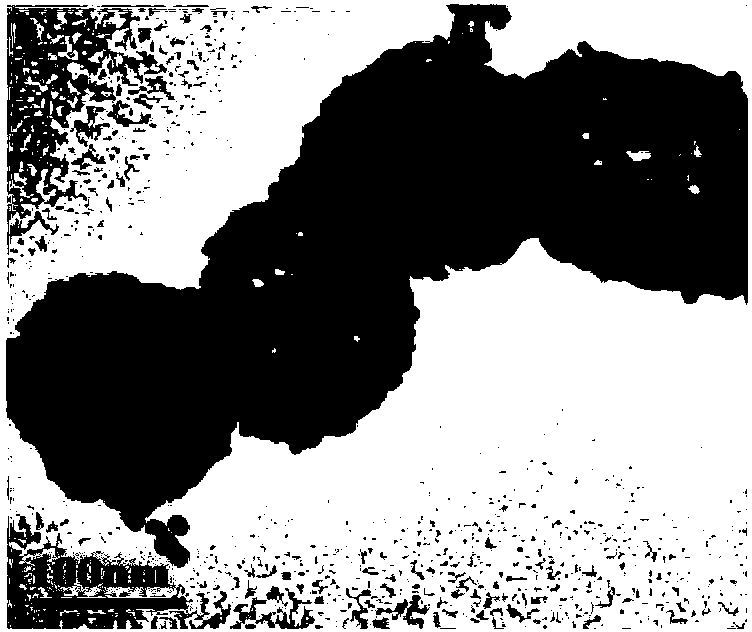
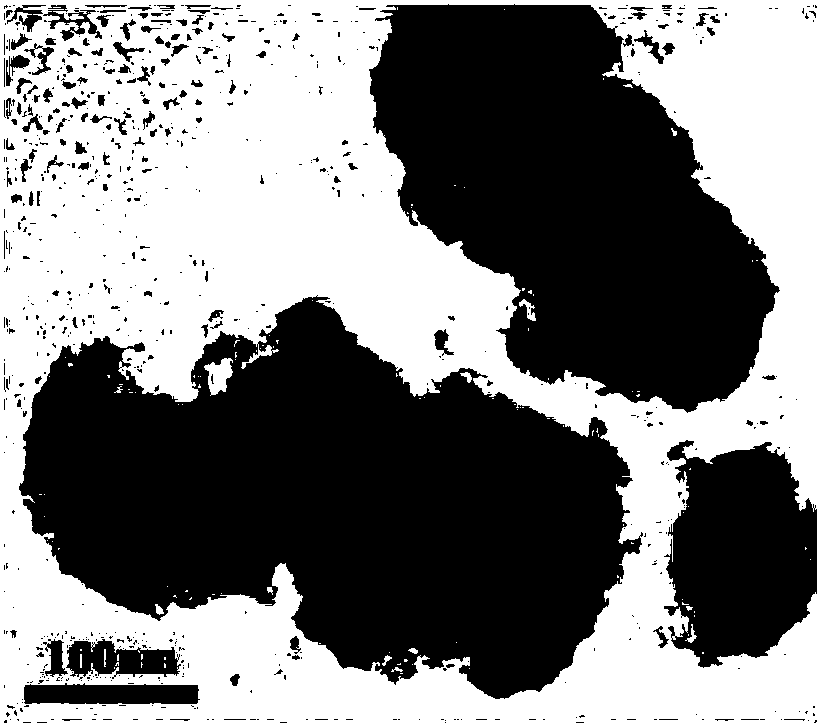
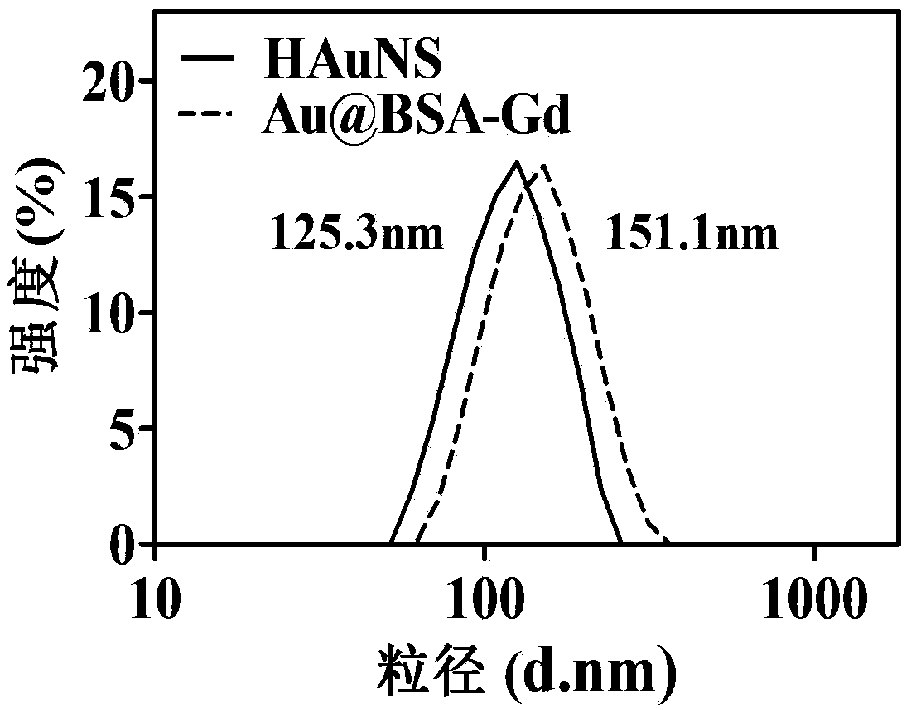
Image
Examples
Embodiment 1
[0044] The preparation method of the hollow gold nanoshell loaded with BSA-gadolinium ion complex comprises the following steps:
[0045] (1) Mesoporous silica nanoparticles (mSiO 2 ) preparation:
[0046] A. Take 0.1g of the template agent cetyltrimethylammonium bromide (CTAB) and add it to 50mL of deionized water, heat to 80°C, adjust the pH to 9, add 320μL of tetraethyl orthosilicate (TEOS) and 80μL of N-(3-trimethoxysilylethyl)ethylenediamine (APTES), add 3mL ethyl acetate, react for 3 hours, centrifuge, and wash the precipitate 4 times with absolute ethanol;
[0047] B. Add 50 mL of absolute ethanol to the precipitate obtained in step A, heat at 60° C. to remove the template agent hexadecyltrimethylammonium bromide, centrifuge, and wash the precipitate 4 times with absolute ethanol;
[0048] C. Repeat step B 4 times to obtain mesoporous silica nanoparticles (mSiO 2 );
[0049] (2) Aminated mesoporous silica nanoparticles (NH 2 -mSiO 2 ) preparation:
[0050] Disper...
Embodiment 2
[0064] Test the photothermal heating curve of Au@BSA-Gd:
[0065] Take phosphate buffer solution with pH=7.4, and take 0.5ml of Au@BSA-Gd aqueous dispersion with different concentrations (17.5, 35, 70 and 140μg / mL) into EP tubes, and use 808nm, 1.5W / cm 2 The power laser was irradiated for 5 minutes, and the temperature change within 0-5 minutes was recorded using a temperature monitor equipped with a thermocouple microprobe (φ=0.5mm). Such as Figure 4 Shown is the photothermal heating curve of Au@BSA-Gd. It can be seen that Au@BSA-Gd can rapidly heat up under light to achieve the purpose of photothermal therapy, and the heating effect is enhanced as the concentration increases.
Embodiment 3
[0067] The survival rate of 4T1 cells in different groups (phosphate buffer group with pH=7.4, laser group, Au@BSA-Gd group and Au@BSA-Gd+laser group) tested by MTT method: 4T1 cells in logarithmic growth phase were taken as 5×10 4 Each well was inoculated in a 96-well plate and divided into four groups: blank phosphate buffer (PBS) group with a pH of 7.4, laser (NIR) group, Au@BSA-Gd group and Au@BSA-Gd+NIR group. After the cells grew for 24 hours, the original cell culture medium was aspirated in each group, 100 μL of 4T1 cell culture medium containing 10 μL of PBS (pH=7.4) was added to the phosphate buffer (PBS) group, and 100 μL of 4T1 cell culture medium was added to the laser (NIR) group. , Au@BSA-Gd group and Au@BSA-Gd+NIR group were added with 100 μL of 4T1 cell culture medium containing Au@BSA-Gd at a concentration of 100 μg / mL. After each group was incubated for 6 hours, the laser group and Au@BSA-Gd+NIR were given 808nm, 1.5W / cm 2 Power laser irradiation for 5 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com